General Neurology
Use of focused ultrasound in neurologic disorders
Jan. 13, 2025
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Worddefinition
At vero eos et accusamus et iusto odio dignissimos ducimus qui blanditiis praesentium voluptatum deleniti atque corrupti quos dolores et quas.
Ankylosing spondylitis is of neurologic interest not only because it may exhibit nervous system manifestations but also because it can be viewed as the articular analog of multiple sclerosis. It is a chronic, generally progressive autoimmune disease that strikes in the prime of life and gives rise to a varied spectrum of articular and, oftentimes, extraarticular symptoms. What is known of its mechanism of disease could contribute to understanding multiple sclerosis and other autoimmune diseases. In this article, the author discusses the clinical manifestations of ankylosing spondylitis, its diagnostic work-up, and its management, including effective, disease-modifying immunomodulatory therapies.
Etymologically, ankylosing spondylitis derives from the Greek roots ankylos (crooked) and spondylos (joint of the back). The name of the disease is evocative of the severely kyphotic posture exhibited by patients with advanced cases of ankylosing spondylitis. Rheumatoid spondylitis, Bechterew syndrome, and Marie-Strümpell spondylitis are alternate names for ankylosing spondylitis. Ankylosing spondylitis is the principal example of the class of disorders known as the seronegative spondyloarthropathies (Table 1).
|
• Ankylosing spondylitis | |
|
| |
Spondyloarthropathies occur in many mammalian species besides man and have even been identified in fossil specimens from three different mammalian orders dating back 30 to 50 million years ago (93). Radiologic studies of Egyptian mummies indicate that some of the pharaohs of ancient Egypt were afflicted with ankylosing spondylitis (39). DNA sequences coding for HLA-B27, a human leukocyte antigen strongly linked to ankylosing spondylitis, have been isolated from a 1000-year-old ankylosed skeleton unearthed in Sweden (59). In 1691, Bernard Connor described a human skeleton with the characteristic features of ankylosing spondylitis (27). Not until the nineteenth century did clinical reports of patients afflicted with ankylosing spondylitis appear, and it was 1930 before the archetypical radiographic findings in this disease were fully appreciated (123).
Ankylosing spondylitis is a progressive inflammatory disorder of the joints that may also affect extraarticular tissues. The hallmark initial symptoms of inflammatory low back pain and joint stiffness usually first appear insidiously in late adolescence or early adulthood, 80% of the time before 30 years of age—afflicting men about twice as often as women (15).
Symptoms are worsened by rest and relieved by activity. Pain may consist of a combination of articular pain and neuropathic pain (132).
Articular pain may spread to the chest and buttocks or may first appear appendicularly (especially in the hips or shoulders), rather than axially in up to one fifth of patients. Initially, radiating pain to one buttock may be mistaken as a symptom of radiculopathy; however, the buttock pain eventually becomes bilateral and is not accompanied by motor or sensory symptoms or signs. Over years to decades, the axial and appendicular articular symptoms become more pronounced. In an unlucky few, the axial articular manifestations may eventually reach the point where the entire spine is effectively fused into a single, markedly kyphotic unit, causing spinal immobility (123).
Extraarticular symptoms of ankylosing spondylitis manifest as the disease evolves. Enthesitis frequently ensues, especially at sites of greater physical stress, and causes patients considerable extraarticular tenderness. Osteoporosis is another widespread extraarticular feature of the disease (36; 15). Patients commonly experience fatigue and may also be prone to other constitutional symptoms such as weight loss and low-grade fevers. Ankylosing spondylitis may affect the eyes, digestive tract, heart, kidney, lungs, nervous system, or skin (Table 2). A systematic literature review and meta-analysis estimated the pooled prevalence of several extraarticular manifestations in ankylosing spondylitis patients: uveitis 25.8%, psoriasis 9.3%, and inflammatory bowel disease 6.8% (106). In a cohort of 216 patients with ankylosing spondylitis followed for an average of 8.3 years, the incidence of new extraarticular manifestations of uveitis, inflammatory bowel disease, or psoriasis was 12.5% (38).
There is a slightly higher risk of malignancy in patients with ankylosing spondylitis; a meta-analysis of 23 studies demonstrated a 14% higher incidence of malignancies in patients with ankylosing spondylitis compared to the general population. Malignancies of gastrointestinal system and hematopoietic system were the most overrepresented (31).
|
Eyes | |
|
• Acute anterior uveitis | |
|
Gastrointestinal | |
|
• Crohn disease | |
|
Heart | |
|
• Aortitis | |
|
Kidneys | |
|
• IgA nephropathy | |
|
Lungs | |
|
• Apical lobe fibrosis | |
|
Nervous system | |
|
• Radiculopathy | |
|
Skin | |
|
• Psoriasis | |
Neurologic complications of ankylosing spondylitis, when carefully sought, are not uncommon. In a small series, 25% of patients with ankylosing spondylitis had neurologic symptoms, or physical examination findings, or both, and 70% had abnormalities on neurophysiologic testing (56).
Due to the relative fragility of the spine, neurologic complications of trauma are more likely to occur in patients with ankylosing spondylitis. Approximately 6% of patients will suffer vertebral fractures, with three quarters of these fractures being associated with trauma (40). These traumatic spinal fractures are most common near the thoracolumbar junction, typically involve both the anterior and posterior portions of the spinal column, and frequently result in the development of chronic Andersson lesions (19).
Direct neurologic manifestations of ankylosing spondylitis include cauda equina syndrome, vertebrobasilar insufficiency, and peripheral neuropathy (112; 99; 116). One review documented 10 of 45 patients with ankylosing spondylitis as having neurologic symptoms and signs (112). Another review catalogued 11 of 100 patients as having neurologic complaints (130). Audiovestibular dysfunction, documented by cochleovestibular testing, occurs more commonly in patients with ankylosing spondylitis than in matched controls (03).
Cauda equina syndrome is a rare (three of 120 patients) (99), but disabling, neurologic consequence of ankylosing spondylitis that occurs only later in the course of the disease (97; 08). It is nontraumatic in origin and is peculiar because it features abundant dural diverticula (08).
There is an increased incidence of vascular disease among patients with ankylosing spondylitis, including a moderately increased incidence of myocardial infarction and stroke (70). Compared to age and sex matched controls, there is a nearly 2-fold higher risk of stroke in young (18 to 45 years of age). The study focused on Taiwanese patients with ankylosing spondylitis (64). However, the increased vascular risk for patients with ankylosing spondylitis is less than among patients with rheumatoid arthritis.
Ankylosing spondylitis is associated with a 1.2-fold higher risk of dementia compared to the age-matched general population (82).
Perhaps the most intriguing neurologic manifestation of ankylosing spondylitis is a constellation of symptoms and signs reminiscent of multiple sclerosis (112; 54; 99; 22; 62; 65; 42). It may simply be that ankylosing spondylitis and multiple sclerosis are comorbid conditions reflecting the well-recognized phenomena that patients with one autoimmune disorder are more prone to other autoimmune disorders, but there is also the possibility that ankylosing spondylitis directly results in central nervous system demyelinating disease that mimics multiple sclerosis (72), similar to what may occur in, for example, systemic lupus erythematosus (22; 34). Further muddying this distinction, several biological drugs used to treat ankylosing spondylitis may induce central nervous system demyelination (see management discussion below).
Reminiscent of multiple sclerosis, ankylosing spondylitis exhibits a wide range of clinical heterogeneity, including the occurrence of spontaneous remissions and relapses (123; 60; 110). In general, life expectancy is normal, and 52% to 85% of patients remain employed (124). Taylor and colleagues published reference charts quantifying validated measures of disease activity, functional impairment, and metrology that allow for population estimates of disease prognosis (110). In the individual patient, hip arthritis, erythrocyte sedimentation rate greater than 30 mm per hour, poor efficacy of nonsteroidal anti-inflammatory drugs, limitation of lumbar axis, sausage finger or toe, oligoarthritis, and onset of disease at 16 years of age or younger are all unfavorable prognostic factors (60). In some patients, the disease may progress to fusion of most of the spine. Whether the clinical course and prognosis differs between men and women is unclear (123), although data suggest that women tend to be more functionally impaired than men (110). A 12-year prospective follow up to the OASIS study demonstrated radiographic progression of ankylosing spondylitis is variable over time even in individual patients but is roughly linear when averaged over the entire study group (85).
Increased spinal rigidity and osteoporosis due to ankylosing spondylitis significantly raise the risk of complications from relatively minor trauma. These trauma-induced complications include spinal fractures, vertebral subluxations, and appendicular fractures (112; 99; 86; 61). In patients with ankylosing spondylitis, the prevalence of osteoporosis is 25%, and the prevalence of vertebral fractures is 10% (28). Patients with ankylosing spondylitis may also experience spontaneous vertebral subluxations (86) or cryptic fractures (24). Spinal fractures and subluxations, in turn, may cause radiculopathies, compressive myelopathies, or even vertebrobasilar insufficiency. In a series, 19 of 103 consecutive patients with ankylosing spondylitis had some degree of myelopathy, and two of these 19 had cervical spinal cord compression severe enough to require cervical fusion (86). In another series, two out of 100 patients with ankylosing spondylitis sustained cervical spine fractures (130). A population-based analysis in Finland disclosed an 11-fold increase in the incidence of traumatic spinal cord injury amongst those with ankylosing spondylitis as compared to the general population (02). Delayed or secondary neurologic deficits following spinal trauma are not uncommon among ankylosing spondylitis patients, underscoring the need to maintain a high index of suspicion in these patients even if they initially present without neurologic compromise (129).
Up to 20% of those patients with ankylosing spondylitis manifesting aortic valvular or aortic root disease develop significant cardiovascular complications, including stroke (92). Posterior circulation strokes may also occur as a complication of atlanto-occipital subluxation (100). Patients with pulmonary involvement occasionally develop fungal infections of the lungs as a complication (123).
Case 1. At 18 years of age a woman developed exercise-responsive low back pain. She later developed iritis and rib cage pain on deep inspiration. Examination disclosed limitation of the lumbar range of motion. Family history was noteworthy for an uncle afflicted with ankylosing spondylitis. A plane radiograph showed mild bilateral sacroiliitis. The erythrocyte sedimentation rate was 5 mm per hour, and the rheumatoid factor was negative; human leukocyte antigen typing demonstrated HLA-B27. She was diagnosed as having ankylosing spondylitis at 21 years of age and was treated with indomethacin and sulfasalazine.
At 24 years of age she suddenly developed pain and pronounced visual loss in the right eye. She was diagnosed with "inflammation of the optic nerve." One year later, her right hand became numb, weak, and clumsy for a couple of months. Neurologic examination was normal except for poor vision and an afferent pupillary defect in the right eye. An MRI of the brain was normal, but a visual evoked response study revealed marked prolongation of the P100 latencies bilaterally, and there were three oligoclonal bands in the cerebrospinal fluid. Over the next few years, she experienced several further episodes of neurologic dysfunction, each of which lasted weeks to months. Symptoms varied and included facial numbness, right lower extremity weakness and numbness, urinary urgency, Lhermitte phenomena, and fatigue. At 30 years of age she developed Crohn disease.
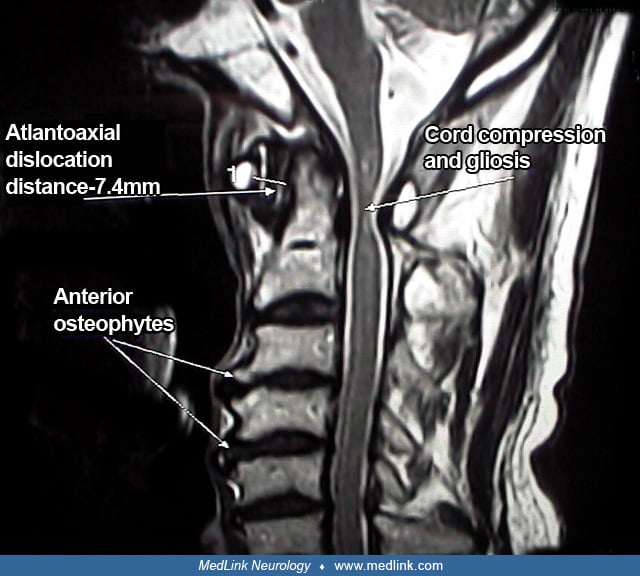
Case 2. An 87-year-old man with a several year history of progressive gait dysfunction fell forward and struck his head on a table. He immediately became quadriparetic. Multiple imaging studies revealed a fracture through a large anterior osteophyte at C4-5 and compression of the spinal cord at that level secondary to a combination of osteophytes and ossification of the posterior ligament. Plane radiographs showed severe “bamboo spine,” and a diagnosis of ankylosing spondylitis was rendered. A C4-5 anterior discectomy with fusion and placement of an Atlantis plate failed to remedy the patient’s myelopathy, and he subsequently required nursing home placement.
Ankylosing spondylitis is primarily an inflammatory disorder of the enthesis: those tissues comprising the bony insertions of ligaments and tendons, the enclosing synovium, and the nearby bone. The ultimate trigger for the inflammatory attack on the entheses is not known but the pathoanatomic, genetic, immunologic, and microbiomic underpinnings of the disease are becoming clearer with each passing year.
The principal pathoanatomical feature of ankylosing spondylitis is sacroiliitis. Syndesmophytes, which are sclerotic protuberances of new or remodeled bone, are another prominent pathoanatomic feature of the disease. Syndesmophytes are preferentially localized to the vertebra. With time, syndesmophytes from adjacent vertebral bodies may fuse to form a bony bridge. In advanced disease, the radiographic finding of "bamboo spine" is the ultimate manifestation of this syndesmophytic bridging.
The histopathological hallmark of ankylosing spondylitis is enthesopathy. The entheses, the sites of ligamentous insertion into bone, are inflamed. T-lymphocytes and macrophages aggregate at these sites, with a predilection for the entheses associated with vertebral bodies and the sacroiliac joints. Bone is remodeled at these sites through an accelerated process of resorption of old bone and formation of new bone, which may represent a pathologic exaggeration of the normal physiologic process of bony remodeling in response to various loads on bone (83).
Spondylitic changes in bone are secondary to immunologically or humorally mediated effects on the G1 domain of the proteoglycan aggrecan component of articular cartilage. Tissues preferentially involved in ankylosing spondylitis, such as the entheses, the annulus and nucleus of intervertebral discs, the sclera and anterior uveal tracts of the eyes, and the media of the aortic wall, are largely composed of collagens (especially type II collagen) containing proteoglycans with G1 domains (83).
Susceptibility to ankylosing spondylitis is, to a great degree, genetically determined. For instance, the risk that a sibling of an ankylosing spondylitis patient will also be afflicted with the disease is 9.2%, whereas the risk in the general population is 0.1% (115). The most noteworthy genetic association involves human leukocyte antigens, especially the human leukocyte antigen class I molecule HLA-B27; indeed, this human leukocyte antigen association with ankylosing spondylitis (with an odds ratio of about 120) is the tightest known among all the suspected autoimmune disorders (113; 131). By comparison, the odds ratio for the association between multiple sclerosis and HLA-DR2 is only 4.1 (113). More than 140 allelic variants of HLA-B27 are known. Different HLA-B27 subtypes are associated with differing degrees of genetic susceptibility for ankylosing spondylitis (109). HLA subtypes HLA-B*2702, HLA-B*2704, HLA-B*2705, and HLA-B*2707 are clearly associated with ankylosing spondylitis, whereas HLA-B*2706, common in Thai and Indonesian people, and HLA-B*2709, common in Sardinians, are not associated with ankylosing spondylitis (55). There is also a dosage effect, with HLA-B27 homozygotes more affected than heterozygotes (88).
Although over 95% of patients with ankylosing spondylitis have HLA-B27, possessing HLA-B27 alone does not necessarily lead to disease. Only 2% of all people with HLA-B27 are afflicted with ankylosing spondylitis (47). Several other loci within the major histocompatibility complex, including other human leukocyte antigen class I sites (the best characterized of which is HLA-B60) and human leukocyte antigen class II and III loci, also contribute to genetic susceptibility for the disease. About 50% of the total genetic variance underlying ankylosing spondylitis is known to be linked to major histocompatibility complex genes, and about 75% of the major histocompatibility complex-linked variance is attributable to HLA-B27 (88). The remaining 50% of the genetic susceptibility is due to at least 25 loci outside the major histocompatibility complex, with more loci being discovered as more genome wide association studies are performed (115). As of 2021, genome-wide association studies have identified over 113 single nucleotide polymorphisms that influence the risk of ankylosing spondylitis (76).
Overall, genetic factors account for about 97% of the population variance in the occurrence of ankylosing spondylitis. Family and twin studies show 75% concordance for ankylosing spondylitis between monozygotic twins, 27% concordance for HLA-B27-similar dizygotic twins, 20% concordance for HLA-B27-similar first-degree relatives, and 13% concordance for HLA-B27-dissimilar dizygotic twins (124; 88). The remaining 3% of the population variance is due to environmental influences. A form of genetic imprinting occurs with ankylosing spondylitis, as the sex of the parent influences the heritability of the disease (22). Whereas the major histocompatibility complex accounts for the preponderance of the genetic risk of developing ankylosing spondylitis, clinical features of the disease (such as degree of functional impairment, age of onset, and measures of disease activity) are determined primarily by nonmajor histocompatibility complex loci on chromosomes 18p, 11p, and 2q (20). Deregulation of several long noncoding RNAs, such as H12, MEG3, or lOC645166, may predispose individuals to ankylosing spondylitis (107).
Of the many components of the immune system that play a role in the pathogenesis of ankylosing spondylitis, three cytokines stand out: tumor necrosis factor, interleukin 17, and interleukin 23. Immunotherapies targeting pathways involving these three cytokines are the basis of disease modifying treatments for ankylosing spondylitis. Interleukin 17 is a proinflammatory cytokine that has been implicated as an important generator of a number of autoimmune diseases, including ankylosing spondylitis and multiple sclerosis (96; 126). Polymorphisms of interleukin 23 and its receptor are associated with ankylosing spondylitis (18).
The composition of the intestinal microbiome is a factor in the pathogenesis of the spondyloarthropathies (45; 128; 17; 52). Intestinal dysbiosis may not only be factor in the genesis of ankylosing spondylitis, but it may also be a factor in its progression (133). Dysbiosis promotes intestinal inflammation, which, in turn, generates interleukin 23, initiating an inflammatory cascade culminating in enthesitis and osteoproliferation (96). The composition of the intestinal microbiome is influenced by HLA-B27 (79; 134) and may be an important factor in predisposing HLA-B27 positive individuals to ankylosing spondylitis. Dietary factors, such as vitamin D levels, influence the microbiome and also exert more direct effects on the immune system such that there is an inverse association between peripheral vitamin D levels and ankylosing spondylitis (33). Several avenues of treatment for ankylosing spondylitis have been proposed, premised on modulating the microbiome: antibiotics, fecal transplantation, probiotics, and diet (105).
Caucasian populations exhibit approximately 0.1% to 0.2% overall prevalence of ankylosing spondylitis. More precisely, the prevalence of the disease parallels the frequency of HLA-B27 in a population (55). In the United States, the prevalence of ankylosing spondylitis has been estimated to be between 0.2% and 0.5% (89). The mean prevalence of ankylosing spondylitis per 10000 is 31.9 in North America, 23.8 in Europe, 16.7 in Asia, 10.2 in South America, and 7.4 in Africa (30). Ankylosing spondylitis, like multiple sclerosis, exhibits a latitudinal geographic variation in its prevalence, with rates of the disease being higher among those residing at the higher latitudes (49).
Expression of the disease is about 2.5 times more common in men than in women, with men being more prone to the spinal form of ankylosing spondylitis and women more prone to peripheral joint form (22). Ankylosing spondylitis tends to present at an older age in women than in men, and women generally have more back pain and fatigue with the disease than do men (94). The prevalence of ankylosing spondylitis in first-degree relatives of patients with ankylosing spondylitis is 80-fold higher than its prevalence in the general population (16). Lower birth order is correlated with increased risk of developing ankylosing spondylitis (07). The racial distribution of the disease depends on the racial distribution of HLA-B27 and HLA-B27 subtypes (55).
No measures are known that can prevent ankylosing spondylitis. Persons afflicted with the disease should take care to avoid trauma because they are at considerably higher risk of developing complications such as fractures, vertebral subluxations, or spinal cord injuries. Primary prevention strategies to avoid the development of spinal cord injury in patients with ankylosing spondylitis include the avoidance of contact sports or potential high-impact activities, installation of home stairway and bathroom railings, and limiting alcohol consumption (51).
Nearly any disorder that prominently features back pain may fall within the differential diagnosis of ankylosing spondylitis, particularly those manifesting inflammatory joint pain (Table 3). Of the disorders listed, the other closely related seronegative spondyloarthropathies are most often confused with ankylosing spondylitis, especially early in the course of the disease. Ankylosing spondylitis is the most common of the seronegative spondyloarthritides (Table 1).
|
• Ankylosing spondylitis |
Inflammatory back pain in adults less than 50 years of age is about 81% specific for spondyloarthropathy if at least two of the following four characteristics are present: morning stiffness lasting over a half hour, improvement with exercise but not with rest, alternating buttock pain, and early morning awakening due to the pain (15). However, this type of back pain is common to all of the spondyloarthropathies and does not serve to sufficiently differentiate ankylosing spondylitis from its congener disorders. Several types of imaging studies are helpful in distinguishing ankylosing spondylitis from clinically similar conditions, such as diffuse idiopathic skeletal hyperostosis or osteitis condensans (14). In 2009 the Assessment of Spondyloarthritis International Society (ASAS) issued a set of diagnostic criteria for ankylosing spondylitis that improved on the old 1984 Modified New York Criteria (Tables 4 and 5) (95).
|
At least one of the following clinical criteria: | |
|
• Low back pain of at least 3 months’ duration improved by exercise and not relieved by rest | |
|
At least one of the following radiographic criteria: | |
|
• Bilateral grade 2 to grade 4 sacroiliitis | |
|
Either of: | |
|
• Sacroiliitis on imaging* plus at least one other SpA feature | |
|
SpA features (mnemonic = SPINEACHE) | |
|
• Sausage digits (dactylitis) | |
|
| |
The seronegative spondyloarthritides can be roughly segregated into two groups: (1) those with predominately axial manifestations and (2) those with predominately peripheral manifestations. The axial spondyloarthritides, in turn, can be divided into those with radiographic findings and those without. Classical ankylosing spondylitis was considered a predominately axial seronegative spondyloarthropathy, with sacroiliitis identifiable on plane radiographs. The introduction of biological therapies has prompted a reappraisal of diagnostic criteria so that patients with earlier or diagnostically challenging disease may access the improved therapies (91). Hence, ankylosing spondylitis may now be diagnosed in a patient with purely peripheral features and no imaging correlate, so long as they fit the 2009 ASAS criteria. Understandably, these broadened criteria further blur the lines between the various types of seronegative spondyloarthritides and have resulted in controversy (84).
Reactive arthritis, as the name suggests, is an autoimmune, asymmetric, mono- or oligoarthritis that occurs in reaction to an instigating infection, most commonly a gastrointestinal or genitourinary infection with shigella, salmonella, campylobacter, or chlamydia species. It has also been reported secondary to COVID-19 infection (77). Most often, reactive arthritis presents as an acute illness that resolves with treatment; less often, it can transform into a chronic disorder. The diagnostic criteria for reactive arthritis require evidence of antecedent infection featuring enteritis or urethritis, which is the aspect of the disorder that most sets it apart from ankylosing spondylitis. Reactive arthritis mostly gives rise to peripheral symptoms.
Most cases of psoriatic arthritis are associated with either current or past psoriatic skin disease. The widely accepted CASPAR criteria for the diagnosis of psoriatic arthritis are different enough from the ASAS criteria for ankylosing spondylitis that the two are usually distinguishable; the only two overlapping criteria are dactylics and negative rheumatoid factor (111). Psoriatic arthritis could mimic mild, nonradiographic ankylosing spondylitis manifesting with mainly peripheral arthritis and dactylitis.
Enteropathic arthritis is a seronegative spondyloarthropathy that occurs in patients with gastrointestinal disease, usually inflammatory bowel disease (Crohn disease or ulcerative colitis). However, other gastrointestinal disorders, such as celiac disease or Whipple disease, may also be associated. Enteropathic arthritis can be tricky to differentiate from ankylosing spondylitis because both conditions share many of the same extraarticular manifestations, including uveitis, aortic insufficiency, and anomalous cardiac conduction. More confusing still, up to a third of patients with enteropathic arthritis have sacroiliitis, and 10% of patients with ankylosing spondylitis are afflicted with chronic inflammatory bowel disease (80).
The diagnosis of ankylosing spondylitis depends primarily on clinical criteria. The key clinical criteria are inflammatory lumbosacral pain and a family history of ankylosing spondylitis (36). The modified Schober test for back movement and standardized chest expansion measurements, when compared to reference values adjusted for age and sex, is helpful in determining if the modified New York criteria are met. Plane radiographs of the pelvis are the most straightforward technical study and, in patients with early to moderate ankylosing spondylitis, will typically demonstrate sacroiliitis (124). In more advanced cases, complete fusion of the sacroiliac joint will be seen. By the time sacroiliitis is seen on plane radiographs, however, ankylosing spondylitis has usually been present for a number of years (36; 69).
Gadolinium enhanced MRI may allow earlier detection of the inflammatory changes seen in the sacroiliac joints and along the spine (09). Indeed, MRI has essentially supplanted plane radiographs in current research trials (06). Short-tau inversion recovery fat-suppression and gadolinium enhanced MRI of the sacroiliac joint discloses subchondral bone marrow edema, synovitis, and capsulitis (69) and can allow for earlier diagnosis of ankylosing spondylitis. Whole body MRI can achieve 69% sensitivity and 94% specificity for diagnosing ankylosing spondylitis (127). With the advent of disease modifying therapies, establishing the diagnosis early now has long-term therapeutic implications (36). Leading experts advocated for a revision in the criteria for the definitive diagnosis of ankylosing spondylitis, arguing that the availability of tumor necrosis-blocking therapies mandates earlier diagnosis and that earlier diagnosis will require giving greater weight to the MRI manifestations of ankylosing spondylitis (101). This led to the proposal of a new set of classification criteria for spondyloarthropathies put forth by the Assessment of Spondyloarthritis International Society (ASAS) that emphasize earlier diagnosis and include patients with MRI findings of sacroiliitis but without the traditional plane radiographic findings of axial spondylitis (87). Both the European League Against Rheumatism and the ASAS recommend that both conventional radiography and MRI be used for the initial diagnosis of ankylosing spondylitis as well as monitoring the disease course and treatment response (57).
Laboratory testing is not usually helpful in diagnosing ankylosing spondylitis: no specific laboratory markers exist, acute phase reactants correlate poorly with the disease, and even synovial biopsy/fluid analysis is nonspecific (36). Routine screening for HLA-B27 is not recommended; the test’s value depends on the pretest probability of disease that includes considerations such as the family history and ethnicity of the patient. HLA typing is most useful in patients with good clinical criteria for the diagnosis but who lack imaging (MRI or plane radiograph) evidence for ankylosing spondylitis (36; 15).
The management of ankylosing spondylitis has been revolutionized by the introduction of biological disease-modifying pharmacotherapies, the first of which appeared in the early 21st century. The efficacy of the biological disease-modifying antirheumatic drugs (bDMARDs) prompted the specification of defined therapeutic targets, with five overarching principles guiding treatment (Table 6).
|
• The treatment target must be based on a shared decision between the patient and rheumatologist. | |
|
• Treatment to target by measuring disease activity, and adjusting therapy accordingly, improves outcomes. | |
|
• Spondyloarthritides are multifaceted diseases; the management of musculoskeletal and extra-articular manifestations should be coordinated, as needed, between the rheumatologist and other specialists. | |
|
• The goals of treating patients with axial spondylarthritis are to optimize long-term health-related quality of life and social participation through the control of signs and symptoms, prevention of structural damage, normalization or preservation of function, avoidance of toxicities, and minimization of comorbidities. | |
|
• Abrogation of inflammation is important to achieve these goals. | |
|
| |
Conventional therapies may be used for patients with milder or more responsive cases of ankylosing spondylitis. Those with more pronounced symptomatology or refractory disease require a combination of conventional therapies plus disease-modifying antirheumatic drugs (108). Conventional therapies include exercise, physical therapy, nonsteroidal antiinflammatory medications, and the conventional disease-modifying agent, sulfasalazine. Appropriate exercise is the mainstay of treatment in ankylosing spondylitis. Consistent, rather than intensive, exercise is most beneficial (123). Nonsteroidal antiinflammatory drug therapy is the first line of pharmacological treatment for ankylosing spondylitis; this may be supplemented with muscle relaxants, analgesics, sulfasalazine, and low-dose corticosteroids (114). Cyclooxygenase-2 specific inhibitors, such as celecoxib or etoricoxib, may be superior to conventional nonsteroidal antiinflammatory drugs in the treatment of ankylosing spondylitis (114; 119). A variety of other agents have been tried, including methotrexate, pamidronate, D-penicillamine, and gold, but their utility is not supported by scientifically rigorous clinical trials (25). In refractory cases, cyclosporine, cyclophosphamide, and intravenous methylprednisolone have been attempted (114; 26).
Biological disease-modifying antirheumatic drugs for ankylosing spondylitis include antitumor necrosis factor agents and inhibitors of interleukin 17, and Janus kinase inhibitors. Multicenter, placebo-controlled, double blind, randomized trials have demonstrated the efficacy and safety of several anti-tumor necrosis agents including: infliximab (12; 118; 41), etanercept (48; 29), adalimumab (102), golimumab (50; 11; 121), and certolizumab (58) in the treatment of ankylosing spondylitis. These disease modifying therapies interfere with the deleterious effects of tumor necrosis factor alpha (71; 114). Infliximab is a chimeric monoclonal antibody directed against tumor necrosis factor alpha; etanercept is a recombinant fusion protein of the tumor necrosis factor alpha p75 receptor and IgG1 Fc (71; 114); adalimumab and golimumab are human monoclonal antibodies directed against tumor necrosis factor alpha (26; 102; 50). Disease-modifying antirheumatic drugs are more effective than standard first-line therapies (108).
An international consensus statement recommends the use of either infliximab or etanercept for the treatment of patients with active ankylosing spondylitis whose disease cannot be satisfactorily managed conventionally (13). Another international task force recommends using biologics to “treat to target,” aiming for specific improvements in surrogate markers of the disease (103). The efficacy of disease-modifying biologics in ankylosing spondylitis can be assessed clinically and also by means of inflammatory biomarkers (especially C-reactive protein and serum amyloid A protein) and MRI characteristics (125; 32). Patients treated with tumor necrosis factor inhibitors may develop antibodies to these agents and, as a result, may suffer decreased efficacy of the treatment (04). Patients who are intolerant of or have insufficient benefit from one antitumor necrosis factor therapy can benefit from switching to a different tumor necrosis factor inhibitor (63). Longer-term treatment with antitumor necrosis factor agents results in continued benefit over time (35) without the emergence of any significant new safety issues (21). It is possible to safely reduce the dose of tumor necrosis factor inhibitors in clinically stable patients with ankylosing spondylitis (43). The effectiveness of antitumor necrosis factor therapies can be assessed both clinically and radiographically. Whole body MRI has been shown to be more sensitive than clinical examination alone in identifying inflammatory lesions (53).
Caution is necessary in using antitumor necrosis factor alpha therapy because of possible adverse effects, including both central and peripheral nervous system demyelination syndromes resembling optic neuritis, multiple sclerosis, multifocal motor neuropathy with conduction block, and both acute and chronic inflammatory demyelinating polyradiculoneuropathies (74; 90; 78; 117). Compared to placebo or no treatment, antitumor necrosis factor agents increase the risk of infections overall by 20%, of serious infections by 40%, and of tuberculosis by 250% (73). The risk of opportunistic infection is somewhat increased in those treated with these therapies (26). Antitumor necrosis factor alpha therapy can also lead to aseptic meningitis (23), severe neutropenia, thrombocytopenia, or reactivation of latent tuberculosis (41). Patients with known demyelinating disease or significant heart failure must avoid tumor necrosis factor alpha antagonists (26). On the other hand, antitumor necrosis agents have not been shown to increase the risk of cancer (10).
Inhibitors of the proinflammatory cytokine interleukin 17A have been proven to be safe and effective disease-modifying treatments for ankylosing spondylitis. These include secukinumab (05) and ixekizumab (120). The cost effectiveness of secukinumab for the treatment of ankylosing spondylitis within the National Health Service of the United Kingdom was affirmed in a pharmacoeconomic analysis (37). Agents targeting interleukin 17A may be particularly useful in treating patients with inadequate responses to TNF inhibitors.
The Janus kinase inhibitors, tofacitinib and upadacitinib, are FDA-approved to treat ankylosing spondylitis in patients refractory to or intolerant of TNF inhibitors (81; 122).
|
First-line therapies | |
|
• Diet | |
|
Second-line therapies | |
|
• Adalimumab | |
|
Adjunctive therapies | |
|
• Analgesics | |
Localized arthritic manifestations can be treated with focal injections of corticosteroids. In certain instances, it is necessary to replace joints surgically. In even more particular circumstances, spinal surgery may be required (123). Focal radiation therapy is now seldom used.
Neurologic outcome following fracture of the ankylosed spine can be improved with surgical intervention (98; 24). Surgical treatment is preferable to medical management alone for cauda equina syndrome associated with ankylosing spondylitis (01).
Ankylosing spondylitis does not reduce fertility, imperil pregnancy, or adversely affect the fetus (46). A review of 21 ankylosing spondylitis pregnancy studies found that disease relapse occurred in 20% to 80% of pregnancies and in 30% to 100% of postpartum women (75). Back pain and stiffness do not improve during pregnancy (68). Regulatory T cells in pregnant women with ankylosing spondylitis show altered cytokine secretion patterns when compared to healthy pregnant women, and this alteration in T regulatory function can persist into the postpartum period (44).
The more advanced cases of ankylosing spondylitis require extra care in positioning and support in preparation for surgery as the susceptibility to compressive neuropathies is increased. It may be necessary to use special techniques for inhalational induction of anesthesia in patients with severe ankylosing spondylitis due to marked limitation of cervical mobility or mouth opening (67). If patients are taking aspirin or sizable quantities of nonsteroidal anti-inflammatory drugs, then they ought to be instructed to discontinue these medications several days prior to surgery.
All contributors' financial relationships have been reviewed and mitigated to ensure that this and every other article is free from commercial bias.

Walter G Carlini MD PhD
Dr. Carlini of Providence Medical Group - Medford Neurology has no relevant financial relationships to disclose.
See Profile
Amy A Pruitt MD
Dr. Pruitt of the University of Pennsylvania School of Medicine has no relevant financial relationships to disclose.
See ProfileNearly 3,000 illustrations, including video clips of neurologic disorders.
Every article is reviewed by our esteemed Editorial Board for accuracy and currency.
Full spectrum of neurology in 1,200 comprehensive articles.
Listen to MedLink on the go with Audio versions of each article.
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
General Neurology
Jan. 13, 2025
General Neurology
Jan. 13, 2025
Neuro-Ophthalmology & Neuro-Otology
Jan. 08, 2025
Neuro-Ophthalmology & Neuro-Otology
Jan. 07, 2025
General Neurology
Dec. 30, 2024
General Neurology
Dec. 13, 2024
General Neurology
Dec. 13, 2024
Neuromuscular Disorders
Dec. 09, 2024