Movement Disorders
Acquired hepatocerebral degeneration
Jan. 20, 2025
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Worddefinition
At vero eos et accusamus et iusto odio dignissimos ducimus qui blanditiis praesentium voluptatum deleniti atque corrupti quos dolores et quas.
The author discusses the concept of ataxia, identifies clinical features useful in evaluating patients with ataxia, and highlights the broad range of disorders that can present with ataxia as a prominent feature. He presents the differential diagnosis, treatment, and management of ataxic conditions, along with the latest information about the most recently discovered forms of hereditary ataxia.
Ataxia is both a neurologic symptom and a sign of incoordination derived from the Greek verb tassein, meaning “to arrange” or “put in order.” Ataxic movements are poorly organized and usually relate to dysfunction of the cerebellum or its numerous connections with other brain regions (43). The Greek physician Herophilus recognized the cerebellum as a distinct brain division, but early studies of cerebellar anatomy and function were not performed until the 17th and 18th centuries (29). The first extensive clinical studies that defined cerebellar syndromes included World War I soldiers who received gunshot wounds to the head (56). Comparative studies between human and animal cerebelli helped define the function of the various cerebellar regions.
Many classification schemes have been developed for hereditary and degenerative disorders that share ataxia as a prominent presenting feature (134). Numerous new genes and their protein products have been identified since the early 2000s, and they should lead to a greater understanding of the pathophysiology and treatments for these diseases in the years ahead.
The term “olivopontocerebellar atrophy” is widely used without clear consensus on specific diagnostic or prognostic implications (10). Many cerebellar disorders were originally classified using clinical features, such as "olivopontocerebellar ataxias, types I to V," or pathological descriptions such as "spinopontine atrophy" or "cortical cerebellar degeneration" (74). These previous classification schemes built on clinical and pathological findings are being replaced by genetic classification based on identification of responsible gene mutations.
Although spasticity can be seen in several types of spinocerebellar ataxias (see Table 1), the term “spastic ataxia” is also occasionally used for the combination of spasticity and ataxia (23). The best known example of Charlevoix-Saguenay ataxia is inherited by autosomal recessive transmission due to sacsin gene (SACS/ARSACS) mutations (35). The first locus for autosomal dominant hereditary spastic ataxia (SAX1) has also been mapped (86). A case of atypical ARSACS in a Japanese patient with ataxia and polyneuropathy but no spasticity has been reported due to a novel compound mutation (p.Lys4326Glu and p.Leu1412Lysfs*16) in the SACS gene (01).
Ataxia is both a neurologic symptom and a sign seen only in association with movement or with an attempt to maintain position against a deflecting force such as gravity. Limb and gait ataxia are both common, but incoordination can also affect speech articulation, swallowing, vision, and truncal movements. Like all movement disorders, ataxia can be described in terms of its location, amplitude, frequency (severity), modifying factors, course over time, and associated neurologic and non-neurologic symptoms. The differential diagnosis of ataxia includes a large number of neurologic disorders, so efficient evaluation requires a careful history and a thorough physical and neurologic examination to place ataxia in its appropriate context. Various rating scales such as the Scale for Assessment and Rating of Ataxia (SARA), the Ataxia Functional Composite Scale (AFCS), and the International Cooperative Ataxia Rating Scale (ICARS) have been developed as sensitive instruments to assess ataxia-related disability reproducibly (05; 146).
Critical features that should be sought out in the patient’s history include age, gender, mode of onset (sudden, gradual, or chronic), lateral distribution (unilateral vs. bilateral), location (eyes, bulbar, limbs, or trunk), and progression (episodic, stable, or progressive). Specific symptoms that people with ataxia may develop include an unstable stance (much like when standing on a ship or boat), increased body sway, side-to-side truncal movements, blurred or bouncing vision (oscillopsia) due to abnormal eye movements, a need for back support when sitting, and widening of stance when standing or walking. The wide range of ataxic etiologies requires a full review of additional neurologic symptoms, including confusion, memory loss, headache, personality changes, seizures or convulsions, double vision, vision loss, weakness, numbness, tingling, itching, hiccups, seizures, hearing loss, tinnitus, voice changes, and word-finding difficulty. Complaints of dizziness should be carefully distinguished among lightheadedness or faintness, rotational vertigo ("room spinning"), rocking movements, and the sense of imbalance described above. The presence of any systemic illness should be noted, but especially diabetes mellitus, known malignancy, human immunodeficiency virus (HIV), cystic fibrosis, and biliary disease. Special inquiries should also be made for autoimmune diseases such as systemic lupus erythematosus, Sjögren syndrome, autoimmune hepatitis, Hashimoto thyroiditis, Graves disease, and celiac disease. Specific physical symptoms to inquire about include significant weight change, gastrointestinal symptoms, frequent infections, jaundice, and other skin changes. Previous trauma, known episodes of illness, prior or current use of medications (especially those that exert effects on the nervous system), and exposure to CNS toxins are all important considerations.
The age of onset can readily shorten a long list of causes. Ataxia of childhood onset is more likely to include congenital, infectious, or metabolic causes and posterior fossa tumors. Adult-onset ataxia during the 20s, 30s, and 40s is more frequently due to demyelinating disease such as multiple sclerosis or sporadic or hereditary ataxias. Ataxia in adults over the age of 50 may indicate ischemic or hemorrhagic stroke or posterior fossa or parietal lobe lesions, but sporadic or hereditary ataxias are also common.
An acute time course of hours to days is suggestive of vascular, traumatic, toxic, or metabolic causes of ataxia. Subacute ataxia presenting over weeks to months often indicates inflammatory, infectious, or paraneoplastic causes of gradual onset and progression. Chronic ataxia over months to years is most frequently genetic, paraneoplastic, or neurodegenerative, although there is often considerable overlap with subacute causes.
A comprehensive physical examination should specifically include orthostatic blood pressure measurements as evidence of autonomic dysfunction. Thyroid assessment should make note of pale or sallow appearance, puffiness or edema of the face, proptosis or lid lag of the eyes, hoarseness of voice, and brittle fingernails and hair in addition to palpable enlargement of the thyroid gland. Abnormalities of cardiac, musculoskeletal (including scoliosis, pes cavus, and hammertoes), and dermatologic examination should all be duly noted. Funduscopic examination should elicit specific evidence of pigmented retinopathy, macular degeneration, optic atrophy, retinal angiomata or telangiectasias, and cataracts. Formal ophthalmologic testing for Kayser-Fleischer rings is indicated in ataxic patients with undiagnosed liver disease, psychosis, tremor, dystonia, or dysphagia.
Neurologic examination should focus on several key features related to cerebellar and sensory dysfunction, including bulbar dysfunction, dysdiadochokinesia, dysmetria, excessive rebound, postural and kinetic limb tremor, rubral tremor, truncal titubation, nystagmus, impaired visual fixation, and gait disturbance.
Bulbar dysfunction includes both dysphagia (impaired coordination of swallowing) and dysarthria (impaired articulation of vocalized speech). Although the term "bulb" refers to the medulla oblongata, dysarthria and dysphagia are poorly localized in the brain, so numerous isolated lesions could result in these disturbances. Pronunciation of the phonemes "T" (primarily lingual), "P" (primarily labial), and "K" (primarily pharyngeal) can distinguish between these broad categories of dysarthria: ta-ta-ta, pa-pa-pa, and ka-ka-ka.
Dysdiadochokinesia refers to impaired coordination or breakdown of alternating movements. Rapid alternation is more likely to identify these breaking points than slower, careful execution of movements. Each upper limb can be tested individually by rapid repetitive pronation and supination of either hand. Each lower limb can be tested individually by rapid repetitive tapping of the heel and then the toes of either foot. Dysdiadochokinesia of articulation involves impaired alternation between distinct syllables even if syllables could individually be articulated effectively. For example, repetition of the phrase "Topeka, Topeka, Topeka" involves alternation between the different phonemes "T", “P,” and "K" described above. The phrase "scanning speech" is another way to describe the noticeable pauses between articulations of distinct syllables.
“Dysmetria” is a broadly used term indicating the inability to judge appropriate distance or measurement. Finger-nose-finger or finger-chin-finger maneuvers (with the eyes open) and finger-to-nose, finger-to-chin, and past-pointing maneuvers (with the eyes closed) are simple clinical assessments of limb overshoot or undershoot. Kinetic tremor involves rhythmic contraction of reciprocally innervated agonist and antagonist muscles noted with continuous movement of an affected body part. Kinetic tremor has a sinusoidal appearance and tends to increase in amplitude as the target is approached. Thus, some authors use the term “dysmetria” to imply inaccuracy (the movement ends off target due to measurement error), whereas kinetic tremor can imply imprecision (the process of movement demonstrates overshoot and undershoot). Rubral or midbrain tremor is a coarse, slow tremor seen at rest, with postural change or with limb movement in patients with disruption of connections to and from the red nucleus in the midbrain, including cerebellar connections.
Excessive rebound is tested by instructing a patient to extend the arms in front and not allowing the examiner to bear down force on the arms. If either or both arms disproportionately elevate in rebound, this "loss of check" implies hypermetria due to difficulty alternating from arm extensor activity to contraction of arm flexor muscles. Similarly, routine strength testing of elbow flexors in patients should involve placement of the examiner's other hand between the patient's arm and the patient's face to prevent excessive rebound resulting in punching the patient's own face.
Examination of eye coordination in cooperative patients includes observation of nystagmus, testing of impaired fixation, eliciting saccades, and identifying smooth pursuit abnormalities. The patient should be asked to fixate on the examiner's nose. Square-wave jerks are inappropriate horizontal saccades whereby the eye leaves the target and then a corrective saccade returns the eye to the target. Voluntary or volitional saccades involve alternating between the examiner's finger or pen on command (held at least 15 degrees laterally from the nose) and then back to the examiner's nose on command. These measurements of ocular dysmetria should be tested in all four cardinal directions. Hypermetric saccades describe overshoot of eye movements whereas hypometric saccades describe eye movements that fall short of the target. Saccadic intrusions that interrupt smooth pursuit movements can also be a nonspecific sign of brainstem or cerebellar disease. Some patients can also demonstrate apraxia of eye movements with difficulty initiating gaze toward one or more of the four cardinal directions on command ("look to the left...now the right").
Truncal titubation refers to sway of an unsupported sitting or standing trunk. Gait disturbance can include a widened stance to compensate for impaired balance, ataxic lurching toward one or both sides, and difficulty performing tandem gait (one foot placed in front of the other without assistance). The Fisher maneuver involves rapid tapping of the index finger to the interphalangeal joint of the ipsilateral thumb, and dysmetria indicates cerebellar pathology. The Romberg maneuver involves narrowing the stance such that one’s legs and feet are touching, if possible. Inability to perform this maneuver with the eyes open is suggestive of cerebellar disease. If balance is maintained successfully, then the maneuver may be "sharpened" by closing the eyes, thereby removing visual input. Subjective sensations of sway should be noted along with objective loss of balance, either of which suggests sensory ataxia from dorsal column or sensory nerve dysfunction. Extension of the arms with supinated hands during this maneuver can help identify "pronator drift" of the upper limb as subtle evidence of upper motor neuron weakness. Because Friedreich ataxia and ataxia with vitamin E deficiency involve degeneration of both the cerebellum and dorsal spinal cord, these disorders may still demonstrate the presence of Romberg sign. Associated neurologic signs and symptoms should be identified, including headache, dystonia, parkinsonism, spasticity, neuropathy, and autonomic dysfunction.
Limb hypotonia and pendular reflexes may be due to cerebellar lesions, particularly when one hemisphere is affected, leading to asymmetric ipsilateral findings. Focal cerebellar lesions can be difficult to separate from associated brainstem lesions and may present with headache, vomiting, blurry vision, double vision, vertigo, lightheadedness, hearing loss, tinnitus, or other cranial nerve abnormalities. Recognition of brainstem or other neurologic symptoms seen in association with ataxia or cerebellar symptoms is crucial due to the risk of acute brainstem compression or transtentorial herniation.
The family history should be reviewed extensively as many patients have undiagnosed blood relatives. Relatives from at least three generations should be evaluated whenever possible. Specific questions should be asked regarding the possibility of consanguinity. Because certain hereditary ataxias have been more commonly described in certain ethnic groups, discussion of ancestry can be useful if one’s heritage is known. Genetic penetrance and variability in clinical manifestation of these diseases among family members can also make it difficult to suspect a similar pathophysiologic process. The existence of a similarly affected parent suggests a dominantly inherited disease, whereas multiple affected siblings without an affected parent would be more typical of a recessively inherited disorder. The presence of multiple affected males in a family related through clinically unaffected or minimally affected females is the hallmark of X-linked inheritance. These disorders can include fragile X-tremor ataxia syndrome (FXTAS) and adrenoleukodystrophy. Family history inquiries should also include questions about autoimmune disease because these disorders may cluster due to underlying immune-mediated dysfunction. Many patients are not familiar with examples of autoimmune disease, so specific disorders such as lupus, Hashimoto thyroiditis, Graves disease, rheumatoid arthritis, Sjögren syndrome, or other specific conditions should be mentioned by name when appropriate. Paraneoplastic ataxia, as well as nonneoplastic autoimmune ataxia, may be a consequence of antiglutamic acid decarboxylase and anti-contactin-associated protein 2 (CASPR2) antibodies (09; 65). Metabotropic glutamate receptor 2 antibody (mGluR2-Ab) in two patients with paraneoplastic ataxia was reported by Ruiz-Garcia and colleagues (114). One of these cases had full resolution with intravenous methylprednisolone and immunoglobulin. Table 1 provides an outline of hereditary ataxias.
Although sporadic versions of most degenerative ataxias exist, the absence of a family history does not entirely exclude the possibility of hereditary disease. As is commonly seen in genetic disorders, some patients are unaware of their true birth status (adoption or nonpaternity may not have been explained), a parent may have died young prior to developing symptoms, or an affected parent may simply be unaware or in denial of symptoms. Recessive disorders typically appear de novo; a hereditary disorder is not often suspected until a second family member develops a similar disease. Even sporadic cases of ataxia do not exclude the possibility of autosomal dominant inheritance. A child of asymptomatic parents may also develop symptoms before the parents due to the phenomenon of genetic anticipation. If the family history is positive, examination of several affected family members can allow the clinician to develop a "family phenotype," which is often broader than the phenotype of a particular individual at a single point in time.
|
U.S. frequency |
Age of onset (years) |
Duration |
Distinguishing features | |
|
SCA-1 |
5%-10% |
30-40 |
10-20 years |
neuropathy, pyramidal signs |
|
SCA-2 |
15% |
20-40 |
5-30 years |
slow saccades, paucity of gaze-evoked nystagmus and dysmetric saccades, neuropathy, dementia |
|
SCA-3 |
15%-25% |
30-40 |
10-15 years |
pyramidal signs, parkinsonism, muscle atrophy, neuropathy, saccadic intrusions |
|
SCA-4/31 |
rare |
30-50 |
normal lifespan |
sensory axonal neuropathy (pure ataxia in one family) |
|
SCA-5 |
rare |
variable |
several decades |
early onset, pure cerebellar, slow course |
|
SCA-6 |
15% |
variable |
several decades |
pure cerebellar ataxia, sometimes episodic, mild neuropathy, prominent nystagmus with downbeat nystagmus induced by head shaking |
|
SCA-7 |
5% |
20-40 |
1-40 years |
pigmentary retinopathy, blindness, hearing loss |
|
SCA-8 |
2%-5% |
20-40 |
normal lifespan |
dysarthria, mild neuropathy, pyramidal signs, late-onset spasticity |
|
SCA-9 |
-- |
-- |
-- |
-- |
|
SCA-10 |
rare |
36 |
9 years |
seizures |
|
SCA-11 |
rare |
20-30 |
normal lifespan |
pure cerebellar, hyperreflexia, slow course |
|
SCA-12 |
rare |
20-30 |
? |
tremor, hyperreflexia, dementia late in course |
|
SCA-13 |
rare |
childhood |
normal lifespan |
mental retardation, pyramidal signs |
|
SCA-14 |
rare |
10-40 |
? |
axial myoclonus, cognitive decline (pure ataxia in some families) |
|
SCA-15 |
rare |
10-50 |
? |
pure cerebellar ataxia, one report of hydrocephalus |
|
SCA-16 |
-- |
20-66 |
? |
head tremor |
|
SCA-17 |
rare |
10-70 |
? |
tremor, parkinsonism, dystonia, chorea, atypical absence and generalized seizures, dysphagia, early dementia, mutism |
|
SCA-18 |
rare |
10-20 |
normal lifespan |
neuropathy, muscle atrophy, sensory loss followed by cerebellar ataxia |
|
SCA-19 |
? |
20-45 |
probably normal lifespan |
mild ataxia, cognitive impairment, myoclonus, postural tremor |
|
SCA-20 |
rare |
19-64 |
? |
palatal tremor, dysphonia, dentate calcification |
|
SCA-21 |
? |
6-30 |
probably normal lifespan |
hyporeflexia, cognitive impairment, rigidity, postural tremor |
|
SCA-22 |
? |
10-46 |
probably normal lifespan |
pure cerebellar, hyporeflexia, slow progression |
|
SCA-23 |
? |
43-56 |
probably normal lifespan |
sensory loss, pyramidal signs, hyporeflexia |
|
SCA-24 |
? |
20-30 |
probably normal lifespan |
pyramidal, myoclonus, horizontal macrosaccadic oscillations, possibly autosomal recessive |
|
SCA-25 |
? |
1-39 |
? |
sensory neuropathy, severe cerebellar atrophy |
|
SCA-26 |
? |
26-60 |
? |
pure cerebellar |
|
SCA-27A |
probably rare |
20-40 |
probably normal lifespan |
tremor, dyskinesia, psychosis, different FGF14 mutation from SCA-27B |
|
SCA-27B |
most common late onset ataxia |
50-60 |
probably normal life span |
pure cerebellar, usually with omnidirectional downbeat nystagmus, sensory deficits (55%), dysautonomia (28%) |
|
SCA-28 |
3% of all SCA patients |
12-36 |
? |
ophthalmoparesis, hyperreflexia, nystagmus, late-onset ptosis |
|
SCA-29 |
rare |
? |
? |
tremor, myoclonus |
|
SCA-30 |
rare |
mid to late life |
slow |
dysarthria, hypermetric saccades, brisk leg reflexes |
|
SCA-31 |
Rare but 3rd most common ADCA in Japan |
59 |
Slow |
Cerebellar syndrome, but 51% of Saucier and colleagues’ case series have deafness, sensory loss, and dysautonomia (116) |
|
SCA-35 |
rare |
40-48 |
5-31 years |
dysarthria prominent, may have cervical dystonia |
|
SCA-36 |
rare, but most frequent in Galicia, Spain |
adult onset |
slow |
hearing loss, ataxic gait more prominent than limb ataxia, dysarthria, slow saccades, tongue fasciculations |
|
Dentato-rubropallidoluysian atrophy (DRPLA) |
rare in U.S. (20% in Japan) |
0-70 |
10-20 years |
chorea, seizures, dementia, myoclonus |
|
Episodic ataxia-1 |
rare |
2-15 |
several decades |
myokymia, exercise-induced attacks lasting seconds to minutes |
|
Episodic ataxia-2 |
rare |
3-50 |
several decades |
nystagmus, vertigo, late-onset ataxia, attacks lasting minutes to hours |
|
Episodic ataxia-3 |
1 family |
1-42 |
? |
vertigo, tinnitus, attacks last minutes to hours |
|
Episodic ataxia-4 |
rare |
? |
? |
nystagmus, vertigo, late-onset ataxia |
|
Episodic ataxia-5 |
1 family |
3-40 |
nystagmus, vertigo, late-onset ataxia, attacks lasting minutes to hours | |
|
Episodic ataxia-6 |
1 family |
6 |
? |
seizures, migraine, alternating hemiplegia |
|
Episodic ataxia-7 |
1 family |
1-20 |
? |
dysarthria, fatigue, attacks lasting hours to days |
|
Episodic ataxia with paroxysmal choreoathetosis |
rare |
2-15 |
? |
limb dystonia, paresthesias, headache, spastic paraplegia |
|
Gerstmann-Sträussler-Scheinker syndrome (GSS) |
rare |
38-70 years |
? |
truncal ataxia, areflexia, lower extremity sensory loss, dementia |
|
Ataxia-telangiectasia like disorder (ATLD) |
rare |
4 |
? |
later onset and milder than ataxia telangiectasia, typically normal total serum immunoglobulin levels, no telangiectasia |
|
Ataxia due to Coenzyme-Q10 deficiency |
rare |
early childhood or late onset |
? |
lipid accumulation on muscle biopsy, dystonia, migraine, tremor, and spasticity |
|
ARSACS (Charlevoix-Saguenay) |
rare, associated with Quebec |
early childhood, late onset form possible |
slow |
pyramidal tract signs and peripheral neuropathy |
|
HSP7 |
2-6/100,000 |
typically 3rd or 4th decade |
slow |
spastic ataxia with dysarthria, nystagmus |
|
Adult onset Alexander disease |
rare |
onset at 12-71 years |
slow |
spastic ataxia, palatal tremor; on imaging, white matter changes, medulla and upper spinal cord atrophy without cerebellar atrophy |
|
Paroxysmal kinesigenic dyskinesia |
rare |
21 year old |
Probably normal |
Episodic ataxia due to C.649dupC pRRT2 mutation |
Spinocerebellar ataxia types 1, 2, and 3 are the most common of the complicated ataxias (those with prominent neurologic symptoms in addition to ataxia). Slowed saccadic eye movements are a hallmark of spinocerebellar ataxia type 2 (139). Spinocerebellar ataxia type 3, also known as Machado-Joseph disease, presents the widest range of symptoms with earlier-onset patients showing prominent spasticity along with ataxia, and patients with older ages of onset sometimes showing prominent parkinsonian features (31). Several review articles characterize the clinical, genetic, and pathologic features of the most common forms of spinocerebellar ataxia (75; 94; 110; 72; 44). SCA3 manifests square-wave jerks and ocular flutter on fixation. Saccadic intrusions appear to be most suggestive for SCA3. Downbeat nystagmus induced by head shaking maneuver is associated with SCA6. SCA2 has a paucity of gaze-evoked nystagmus and dysmetric saccades (68). “Bulging eyes” have been reported more frequently in SCA3 as opposed to SCA1, SCA2, SCA6, and SCA7 (90).
Some hereditary ataxias present with pure cerebellar ataxia, including spinocerebellar ataxia types 4, 5, 6, 10, 11, 14, 15, 16, 22, and 26. Spinocerebellar ataxia type 6 may present with much milder or later onset, sometimes with episodic ataxia at onset or prominent vestibular symptoms and nystagmus as an early finding. Unlike spinocerebellar ataxia types 1, 2, or 3, individuals with spinocerebellar ataxia type 6 tend to lack pyramidal, extrapyramidal, or bulbar symptoms, although a mild neuropathy may be observed (59). Axonal sensory polyneuropathy is also a feature noted in one family with spinocerebellar ataxia type 4. Spinocerebellar ataxia type 7 is associated with retinal degeneration and tends to follow an earlier, more rapidly progressive course than some of the other dominant ataxias.
Mild incoordination and cerebellar atrophy are present in SCA1 and SCA2 carriers prior to the development of the full clinical ataxia phenotype. In addition, gaze-evoked nystagmus is present in pre-symptomatic SCA3 carriers. Pre-symptomatic SCA2 carriers show brainstem atrophy whereas brainstem volume remains normal in SCA6 carriers (61). Based on an analysis of 462 patients with SCA1, SCA2, SCA3, or SCA6 and a median observation time of 49 months, the annual SARA score increase was 2.11 (SE 0.12) in patients with SCA1, 1.49 (0·07) in patients with SCA2, 1.56 (0·08) in patients with SCA3, and 0.80 (0·09) in patients with SCA6. Older age at inclusion and longer repeat expansions were associated with more rapid progression of the SARA score (60).
Friedreich ataxia is the most common autosomal recessive ataxia and should be considered in sporadic individuals without family history or with only a family history seen in distant relatives. Patients typically present with limb and gait ataxia, absent reflexes due to sensory axonal neuropathy, and pyramidal signs, although spasticity and a variety of other neurologic signs have been described since the discovery of the causative gene (109).
The age of onset is most commonly before adolescence, but some patients may have preserved reflexes and present even in their 20s or 30s. It is important to consider this diagnosis in patients with an atypical presentation. Friedreich ataxia involves multiple organ systems and increases the risk for diabetes mellitus, hearing or visual loss, progressive scoliosis, and cardiomyopathy ultimately leading to fatal congestive heart failure. In addition to progressive ataxia, patients may exhibit a variety of hyperkinetic movement disorders, such as kinetic tremor, dystonia, and chorea (58). Friedreich ataxia should be carefully distinguished from ataxia with oculomotor apraxia in which most children and young adults also display evidence of initial choreoathetosis, hypoalbuminemia, and abnormal lipid profiles (88).
Several physical examination findings can often be valuable in suggesting different possible disorders. The absence of these findings, however, does not exclude these disorders completely. Scoliosis is a key feature of Friedreich ataxia, ataxia with oculomotor apraxia types I and II, and Marinesco-Sjögren syndrome. Hearing loss can be found in Friedreich ataxia, Refsum disease, mitochondrial recessive ataxia syndrome, and infantile onset spinocerebellar ataxia syndrome. Cataracts may be seen in cerebrotendinous xanthomatosis and Marinesco-Sjögren syndrome. Retinitis pigmentosa is more characteristic of ataxia with vitamin E deficiency, Refsum syndrome, and abetalipoproteinemia. Optic atrophy is seen in ataxia with oculomotor apraxia (type I only) and infantile-onset spinocerebellar ataxia syndrome. Evidence of hypogonadism can be found in infantile-onset spinocerebellar ataxia syndrome and Marinesco-Sjögren syndrome. CAPOS syndrome (cerebellar ataxia, areflexia, pes cavus, optic atrophy, and sensorineural hearing loss) is in the differential diagnosis but is less likely given 1 in 1 million incidence. A key feature is ataxic encephalopathy in children after a febrile illness. This disorder is caused by ATP1A3 mutation that is different from the mutation causing alternating hemiplegia of childhood or DYT12/rapid onset dystonia parkinsonism (24; 87).
Other common genetic ataxias can be recognized by distinctive clinical features. Episodic ataxia, as the name suggests, is characterized by attacks of incoordination often related to exercise or stress and usually beginning before 20 years of age. These disorders are most commonly the result of autosomal dominant channelopathies caused by mutations in two genes, KCNA1 and CACNA1A (63). Co-occurrence of episodic ataxia type 2 and SCA6 phenotype has been described in a single family tree due to same p.D302N CACNA1A mutation (105). A case of episodic ataxia in a 21-year-old woman due to C.649dupC PRRT2 mutation was reported (78). PRRT2 is typically associated with paroxysmal kinesigenic dyskinesia. Ataxia telangiectasia is a disorder of DNA repair with autosomal recessive inheritance. Additional systemic symptoms include telangiectasias of the skin or conjunctivae, immune deficiency with recurrent infections, and increased incidence of hematologic malignancies. Although dentatorubropallidoluysian atrophy (DRPLA) is uncommon in the United States, a large African-American family located in North Carolina has a similar condition known as “Haw River syndrome.” DRPLA is more commonly seen in those of Japanese descent and affects multiple systems resulting in possible cognitive decline, seizures, myoclonus, and chorea. Seizures can be seen in patients with episodic ataxia type 6 and spinocerebellar ataxia types 10 and 17 (85).
In one study of 1500 patients with ataxia assessed over 20 years in the Sheffield Ataxia Centre, UK, 20% reported family history of ataxia (52). In the cases of sporadic ataxia, 25% were “gluten ataxia” and genetic cause was identified in 156 (13%) of sporadic cases. Other causes included alcohol abuse (12%) and cerebellar variant of multiple system atrophy (11%). Using next-generation screening, genetic mutation or variant was found in 32% of 146 patients tested; the commonest ataxia identified was episodic ataxia type 2 (EA2). A genetic diagnosis was achieved in 57% of all familial ataxias. The commonest genetic ataxias were Friedreich ataxia (22%), SCA6 (14%), EA2 (13%), SPG7 (10%), and mitochondrial disease (10%). The overall diagnostic yield was 63%.
Spinocerebellar ataxia type 15 (SCA15). SCA15 is associated with ITPR1 gene variants. It is known as a classic pure ataxia, but there is a single case report of a hydrocephalus phenotype that improved with ventriculoperitoneal shunting (131).
Spinocerebellar ataxia type 19 (SCA19). SCA19 has been found to be caused by a potassium channel mutation (30). More specifically, mutations involve the KCND3 gene coding for Kv4.3, which is a voltage-gated potassium channel highly expressed in the cerebellum. SCA19 is associated with mild ataxia, cognitive impairment, myoclonus, and postural tremor.
Spinocerebellar ataxia type 21 (SCA21). Spinocerebellar ataxia type 21 is an early onset ataxia associated with cognitive impairment. It has been associated with the TMEM240 transmembrane protein (25).
GAA-FGF14 ataxia/spinocerebellar ataxia 27B (SCA27B). FGF14 gene GAA repeat expansions in the first intron have been in found in six persons with late onset autosomal dominant cerebellar ataxia, and this repeat expansion was confirmed in a larger case control series (102). It produces a pure cerebellar ataxia syndrome, with afferent sensory deficits reported in 55% of patients and dysautonomia in 28% (142). Omnidirectional downbeat nystagmus is commonly seen in SCA27B and is likely due to disruption of the vestibulo-ocular reflex at the cerebellar cortex (124). There was partial improvement in downbeat nystagmus after treatment with 4-aminopyridine (142).
Spinocerebellar ataxia type 35 (SCA35). Spinocerebellar ataxia type 35 (SCA35) is a rare autosomal dominant progressive ataxia with onset in the fourth decade of life. It was first reported in two Chinese families who had mutations in the TGM6 transglutaminase gene (141). Clinical features include onset in the fourth decade of life, disease duration of up to 31 years, prominent cerebellar dysarthria, upper motor neuron findings, cervical dystonia, early gait disturbance, and late upper extremity ataxia.
Spinocerebellar ataxia type 36 (SCA36). SCA36 is an autosomal dominant, adult onset progressive ataxia found to be caused by GGCCTG repeat expansion. It is also called Costa da Morte ataxia based on a coastal area of Galicia, Spain, where it is the most common type of genetic spinocerebellar ataxia (42). Cases were also reported from western Japan, but it is otherwise rare worldwide. The repeat expansion occurs in the NOP56 gene, whose product is a component of the 60S ribosomal subunit.
Ataxia-telangiectasia-like disorder (ATLD). ATLD has been identified to be due to MRE11 mutation. The MRE11 protein forms a part of the MRE11/RAD50/NBS1 (MRN) complex, which is involved in repairing damage from ionizing radiation (129). In contrast to ataxia telangiectasia, these patients have no telangiectasias, they have normal total serum immunoglobulin levels and normal serum alpha-fetoprotein, and they are less sensitive to damage from ionizing radiation. Onset in the first reported cases was at age 3 to 4 years with mildly ataxic gait, dysarthria during teenage years, and vertical nystagmus as well as saccadic pursuit (55).
Gerstmann-Sträussler-Scheinker syndrome (GSS). GSS is an autosomal dominant prion disease that may cause progressive ataxia. GSS caused by the Pro102Leu mutation in the PRNP gene (GSS102) manifests prominent truncal ataxia, absent reflexes, and lower extremity sensory loss as well as dementia in late stages (04). MRI findings include diffuse cerebral atrophy as well as hyperintense cortical diffusion-weighted imaging lesions consistent with prion disease.
Episodic ataxia type 1 (EA1). EA1 is an autosomal dominant disorder with brief attacks of ataxia that last seconds to minutes as well as myokymia between episodes. Sensation of imbalance preceding the attack has been reported (63). Mutations of the KCNA1 potassium channel gene, analogous to the Shaker potassium channel in drosophila, are responsible for the disease. Acetazolamide and avoidance of stress and caffeine may be useful in controlling disease manifestations (22).
Episodic ataxia type 2 (EA2). Brain-specific P/Q-type Ca2+ channel alpha 1-subunit gene, CACNL1A4, mutations causing episodic ataxia type 2 and familial hemiplegic migraine were first reported in 1996 by Ophoff and colleagues (98). Familial hemiplegic migraine involves migraine with aura that includes hemiparesis and possible cerebellar atrophy, whereas episodic ataxia type 2 consists of cerebellar atrophy and attacks of ataxia lasting over an hour with near normal neurologic function except for nystagmus between attacks. Episodic ataxia type 2 was previously called acetazolamide-responsive hereditary paroxysmal cerebellar ataxia (138). Choi and colleagues identified SLC1A3 mutation as another genetic cause of an EA2 phenotype (19).
Episodic ataxia type 2 due to CACNA1A was reported in a Taiwanese family with an older age of onset (third and fourth decade of life) and family history absent for migraines (79). The particular mutation was c.4537G/T, p.1437R/L in exon 27 of the CACNA1A gene. The ataxia responded well to acetazolamide. Intron 19 c.3093A/G mutation leads to aberrant splicing and was found in a woman with classic acetazolamide responsive EA2 phenotype. Her father had the same mutation but was acetazolamide unresponsive (128).
Spinocerebellar ataxia type 6 (SCA6), which may have an episodic phenotype, is caused by CAG repeats in the CACNA1A gene (151).
Ataxia due to coenzyme Q10 deficiency. Horvath and colleagues describe multiple cases of ataxia secondary to mutation in CABC1/ADCK3, which codes for a kinase that modulates Coenzyme Q10 synthesis (57). These patients may have lipid accumulation on muscle biopsy, but they do not have not ragged red fibers. A childhood-onset variant of ataxia as well as milder late onset variant is possible. Spasticity, epilepsy, and exercise intolerance are present to varying degrees. Chang and colleagues recommend ubiquinone titrated from 10 mg/kg/day to 15 mg/kg/day as a 6-month treatment trial (16).
SARS-CoV-2-related acute cerebellar ataxia and myoclonus (ACAM). Individual case reports of SARS-CoV-2-related acute cerebellar ataxia and myoclonus (ACAM) have been reported (48). These cases have an excellent clinical response to intravenous steroids followed by oral steroids, immunoglobulins, or both. Opsoclonus may or may not be present.
Other rare ataxias. Cerebrotendinous xanthomatosis is a disorder of CYP27A1 gene product function, which is sterol 27 hydroxylase. Mutations lead to abnormal brain cholesterol deposits while serum cholesterol level remains normal. Treatment with chenodeoxycholic acid replacement therapy is possible (03). Childhood cataracts and Achilles tendon xanthoma can be diagnostic clues (121).
Abetalipoproteinemia, secondary to microsomal triglyceride transfer protein mutation, can be treated with alpha-tocopherol.
Sensory axonal neuropathy with dysarthria and ophthalmoplegia (SANDO), with onset between 20 and 60 years of age, is caused by POLG mutation and may lead to ataxia with dysarthria, ophthalmoplegia, and myoclonus.
Fatty acid hydroxylase-associated neurodegeneration (FAHN) due to FA2H mutation is in the family of neurodegeneration with brain iron accumulation disorders and may have an ataxic phenotype in addition to dystonia and spasticity.
Mutation in the JNK pathway (CCDC88C) has been found to cause an autosomal dominant form of ataxia (132). A new autosomal dominant spinocerebellar ataxia with linkage to hematologic cytopenias has also been found (17).
The CANVAS syndrome consists of cerebellar ataxia, neuropathy, and vestibular areflexia. Dysautonomia has been reported in 83% of a group of 26 patients with CANVAS (145). Biallelic intronic AAGGG repeat expansion in replication factor complex subunit 1 (RFC1) has been identified as a cause of this syndrome. Cough, vestibular dysfunction, cerebellar involvement, and sensory neuropathy are diagnostic clues for this disorder (20). Persistent cough was seen in three out of five patients from different families, and cough preceded ataxia and neuropathy in two of them as reported by Cakar and colleagues (14).
Aconitase (ACO2) mutation has been found to cause juvenile cerebellar ataxia without retinal involvement, whereas infantile ataxia and retinal degeneration occur with a more severe phenotype (126; 08).
CWF19L1 mutation has been associated with autosomal recessive cerebellar ataxia and atrophy (96). ANO10 mutation can cause adult onset cerebellar ataxia with atrophy, coenzyme Q10 deficiency in muscle with and without ptosis, nystagmus, and spasticity with possible mild clinical improvement with coenzyme Q10 supplementation (07).
Autosomal recessive spinocerebellar ataxia 20 (SCAR20) was found to be due to mutations in the SNX14 gene and includes hearing loss, cognitive disturbance, and progressive coarsening of facial features (125).
PHARC (polyneuropathy, hearing loss, ataxia, retinitis pigmentosa, and early-onset cataract) is a rare autosomal recessive disorder caused by ABHD12 mutations (130).
Autosomal dominant cerebellar ataxia with deafness and narcolepsy (ADCA-DN) phenotype also includes dementia, polyneuropathy, and dysautonomia. This disorder was found to be due to DNMT1 gene mutations (140).
Point mutation in noncoding RNA, RNU12, has been identified as a cause of early-onset cerebellar ataxia. This gene codes for a small nuclear RNA (snRNA), which is a spliceosome component (33).
ATP8A2 mutations may cause recessively inherited ataxia in addition to the better-known syndrome of encephalopathy, intellectual disability, severe hypotonia, chorea, and optic atrophy (49).
RNA polymerase POLR1C mutations can cause young onset ataxia and leukodystrophy with myopia, diffuse hypomyelination, and thalamic and dentate T2 MRI hypointensities serving as diagnostic clues (53).
Young onset ataxia, deafness, and progressive spasticity have been described with KCNJ10 mutations. This gene encodes Kir4.1 potassium channel and is involved in epilepsy, ataxia, intellectual disability, sensorineural deafness, and tubulopathy (EAST/SeSAME syndrome) (89).
VPS13 gene, whose mutations have been identified in chorea-acanthocytosis and rapidly progressive early onset Parkinson disease, can also be involved in spastic ataxia (32).
Mitochondrial cerebellar ataxia, renal failure, neuropathy, and encephalopathy (MCARNE). This is a mitochondrial disorder from the same missense mutation (mitochondrial NADH dehydrogenase 5 [MT-ND5] Asp393Asn) that can also cause mitochondrial encephalopathy and lactic acidosis with stroke like episodes (MELAS). A case report of a 60-year-old male with this mutation presenting as peripheral neuropathy, renal failure requiring transplantation, memory loss, construction apraxia, and severe cerebellar degeneration leading to ataxic dysarthria and appendicular as well as gait ataxia was published by Ng and colleagues (95).
Paraneoplastic ataxia due to anti-Yo antibody (anti-Purkinje cytoplasmic antibody 1/PCA-1). This is the most common cause of paraneoplastic cerebellar degeneration. Dysarthria, ataxia, and vertigo with progressive course are seen clinically. The typical presentation is a female more than 60 years old with presence of gynecological cancer such as breast, uterine, or ovarian cancer. It is rarely reported on lung cancers and in men (54). A single case due to cholangiocarcinoma has been reported (83).
Opsoclonus-myoclonus-ataxia syndrome (OMAS) associated with SARS-CoV-2. There are individual case reports of ataxia as well as opsoclonus-myoclonus-ataxia reported secondary to SARS-CoV-2. In the report by Urrea-Mendoza and colleagues, there was a substantial improvement of myoclonus and ataxia and resolution of opsoclonus at day 24 after diagnosis of SARS-CoV-2 and treatment with methylprednisolone 40 mg daily (133). Przytuła and colleagues report two cases of ataxia myoclonus and reviewed 14 other published cases (106). Twelve of these cases were treated with intravenous immunoglobulin, steroids, or both, and significant clinical improvement was reported within 1 to 8 weeks.
The prognosis of ataxia depends on the underlying diagnosis. Severe unilateral or bilateral cerebellar ataxia, by itself, is not life-threatening, although the tight space in which the cerebellum is contained does not accommodate much swelling or edema. Therefore, acute lesions of the cerebellum accompanied by edema or space-occupying lesions can be life-threatening and require immediate neurosurgical attention. Among hereditary causes of ataxias, the prognosis depends on the specific genetic diagnosis, the associated symptoms in the particular individual, and to an extent, the age of disease onset.
In general, spinocerebellar ataxia types 1, 2, and 3 are conditions that progress over 5 to 20 years with progressive disability related to gait and bulbar dysfunction. In the late stages, the consequences of immobility and dysphagia include weight loss, pneumonia, urinary tract infections, venous thromboemboli, sepsis, and eventually death. Spinocerebellar ataxia type 6 and other pure cerebellar ataxias are associated with milder, prolonged courses and may not shorten the lifespan. Spinocerebellar ataxia type 7 tends to have a more malignant course, often leading to blindness and death within a decade. The prognosis for patients with dominant ataxia without a mutation in one of the known genes can only be based on the family phenotype and may not be accurate. Some patients with dominant ataxias appear to develop a mild dementia although this has not been well-studied. Little information is presently available regarding associated prognosis of the rarer, newly discovered forms of spinocerebellar ataxia.
The course of sporadic olivopontocerebellar atrophies is similar to that of the hereditary olivopontocerebellar atrophies or multiple system atrophy in that they show slow progression over several years leading to a chair-bound or bed-bound state.
The prognosis of Friedreich ataxia is that of a slowly progressive spinal cord disease. Most patients are wheelchair bound within 10 years of onset but are able to live for decades after that time with appropriate assistance. Bowel and bladder control are often impaired as the disease progresses, and dysarthria becomes more prominent making communication more difficult. Although cognitive disturbance was once thought to be atypical in Friedreich ataxia, greater understanding of the cerebellar role in cognition has led to further research in this area. One study showed evidence of decreased motor and mental reaction times, letter fluency deficits, impairment in acquiring and consolidating verbal information, and visuospatial abnormalities compared with controls (143).
A 35-year-old man presented with a history of episodic dizziness, unsteadiness, and loss of coordination that started at 13 years of age. These symptoms lasted from 30 minutes to several hours. He denied any provoking factors that caused his symptoms. Episodes of dizziness and incoordination increased in frequency, and he experienced almost daily episodes at 30 years of age. He also noticed his words slurring during the episodes. He reported poor balance when he did not experience his typical spells. The remainder of his past medical history was unremarkable, and he denied alcohol abuse.
Family history was significant for similar problems experienced by his brother and son. The patient reported that his son’s first episode was provoked by a strenuous physical activity at 8 years of age, and 2 years later, he began to have almost daily attacks of dizziness and unsteadiness that lasted for several hours. His brother had similar age of onset, but his paroxysmal ataxia episodes were less severe and less frequent.
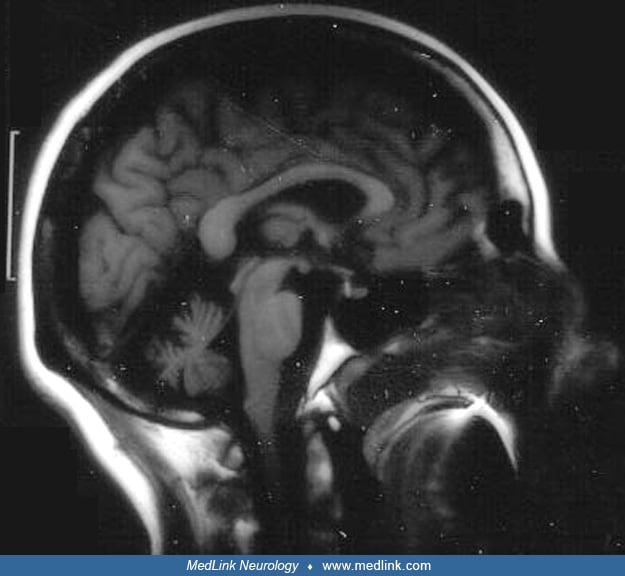
Interictal neurologic examination of the proband demonstrated saccadic pursuit, hypermetric saccades, and mild midline ataxia. His MRI demonstrated moderate atrophy of the superior cerebellar vermis with sparing of other parts of the cerebellum.
DNA testing for spinocerebellar ataxia type 6 was with normal number of CAG repeats in both alleles.
The patient was started on acetazolamide without any clinical change. Later, he was switched to another carbonic anhydrase inhibitor dichlorphenamide, and he reported significant improvement of his symptoms.
More than 780 hereditary conditions where ataxia can be a symptom are listed in the Online Mendelian Inheritance in Man. These disorders can be divided into common dominant and recessive conditions, less common and ill-defined dominant and recessive syndromes, metabolic disorders, mitochondrial disorders, disorders of DNA repair, structural disorders of the cerebellum, ataxia associated with abnormality of the sensory systems (blindness, deafness, or peripheral neuropathy syndromes), and ataxia associated with certain mental retardation syndromes. Table 2 presents the key genetic features of the dominantly inherited ataxias (127; 113).
A wide variety of lesions can also affect the cerebellum, including vascular, infectious, inflammatory, demyelinating, degenerative, toxic/metabolic, and neoplastic, among others.
|
Disorder |
Chromosome |
Gene |
Protein |
Mutation |
Available Testing |
|
SCA-1 |
6p23 |
SCA1 |
ataxin-1 |
CAG expansion |
Yes |
|
SCA-2 |
12q24.1 |
SCA2 |
ataxin-2 |
CAG expansion |
Yes |
|
SCA-3 |
14q21 |
SCA3 |
ataxin-3 |
CAG expansion |
Yes |
|
SCA-4/31 |
16q22.1 |
? |
? |
TGGAA pentanucleotide repeat insertion |
No |
|
SCA-5 |
11q13 |
SPTBN2 |
β-III Spectrin |
inframe deletions and missense mutations (Leu253Pro) |
Yes |
|
SCA-6 |
19p13.1-p13.2 |
CACNA1A |
α-I voltage-dependent calcium channel protein |
CAG expansion |
Yes |
|
SCA-7 |
3p14-p21.1 |
SCA7 |
ataxin-7 |
CAG expansion |
Yes |
|
SCA-8 |
13q21 |
SCA8 |
-- |
CUG expansion of 3' mRNA with incomplete penetrance |
Yes |
|
SCA-9 |
reserved |
-- |
-- |
-- |
-- |
|
SCA-10 |
22q13 |
ATXN10 |
ataxin-10 |
ATTCT expansion in intron 9 |
Yes |
|
SCA-11 |
15q14-q21.3 |
TTBK2 |
tau tubulin kinase 2 |
stop frameshift insertion or deletion mutations |
No |
|
SCA-12 |
5q31-q33 |
PPP2R2B |
protein phosphatase2, regulatory subunit B |
CAG expansion |
Yes |
|
SCA-13 |
19q13.3-q13.4 |
KCNC3 |
missense mutations (Phe448Leu and Arg420His) |
Yes | |
|
SCA-14 |
19q13.4 |
PRKCG |
protein kinase C-γ |
numerous missense mutations |
Yes |
|
SCA-15/16/29 |
3p25-26, 3p26.2 |
ITPR1 |
inositol 1,4,5-triphosphate receptor, type 1 |
Heterozygous deletion of 5’ regions; can extend into neighboring SUMF1 gene |
No |
|
SCA-17 |
6q27 |
TBP |
TATA-box binding protein |
CAG expansion |
Yes |
|
SCA-18 |
7q31-q32 |
? |
? |
? |
No |
|
SCA-19 |
1p21-q21 |
KCND3 |
Voltage gate K+ channel |
T352P, M373I, S290N |
No |
|
SCA-20 |
11 |
? |
? |
? |
No |
|
SCA-21 |
7p15.1-p21 |
TMEM240 |
Transmemb-rane protein |
c.509C>T, c.239C>T,c.346C>T, and others |
No |
|
SCA-22 |
1p21-q23 |
? |
? |
? |
No |
|
SCA-23 |
20p12.2-p13 |
? |
? |
? |
No |
|
SCA-24 |
1p36 |
? |
? |
? |
No |
|
SCA-25 |
2p15-p21 |
? |
? |
? |
No |
|
SCA-26 |
19p13 |
? |
? |
? |
No |
|
SCA-27 |
13q34 |
FGF14 |
fibroblast growth factor 14 |
point mutations (F145S) |
No |
|
SCA-28 |
18p11.21 |
AFG3L2 |
ATPase family gene 3-like 2 |
? |
No |
|
SCA-29 |
3p26 |
? |
? |
probable variant of SCA-15 |
No |
|
SCA-30 |
4q34.3-q35.1 |
ODZ3 (candidate gene) |
Tenascin M3 |
? |
No |
|
SCA-35 |
20p13–12.2 |
TGM6 |
Transglutaminase-6 |
Missense L517W and D327G |
No |
|
SCA-36 |
20p |
NOP56 |
Nucleolar protein |
GGCCTG repeat expansion |
No |
|
SCA-37 |
1p32.2 |
DAB1 (119) |
? |
ATTTC(n) insertion |
No |
|
SCA-38 |
6p12 |
ELOVL5 (26) |
? |
missense G230V and L72V |
No |
|
SCA-40 |
14q32.11-q32.12 |
CCDC88C |
JNK signaling (132) |
R464H |
No |
|
SCA-41 |
4q27 |
TRPC3 (40) |
? |
Heterozygous missense mutation |
No |
|
SCA-42 |
17q21.33 |
CACNA1G (69) |
Voltage gated calcium channel, alpha1 G subunit |
c.5144G>A; R1715H |
No |
|
Dentato-rubropallido-luysian atrophy (DRPLA) |
12p13.31 |
DRPLA |
atrophin-1 |
CAG expansion |
Yes |
|
Episodic ataxia-1 |
12p13 |
KCNA1 |
voltage-gated potassium channel protein |
missense point mutations |
No |
|
Episodic ataxia-2 |
19p13.1-p13.2 |
CACNA1A |
voltage-dependent calcium channel protein, α1 subunit |
point mutations or small deletions (CAG expansion in two families) |
No |
|
Episodic ataxia-3 |
1q42 |
? |
? |
? |
No |
|
Episodic ataxia-4 |
? |
PATX |
? |
? |
No |
|
Episodic ataxia-5 |
2q22-q23 |
CACNB4β4 |
voltage-dependent calcium channel protein, β4 subunit |
? |
No |
|
Episodic ataxia-6 |
5p11-12 |
SLC1A3 |
glial high affinity glutamate transporter protein |
? |
No |
|
Episodic ataxia-7 |
19q13 |
? |
? |
? |
No |
|
Episodic ataxia with paroxysmal choreo-athetosis |
1p |
DYT9 |
? |
? |
No |
|
Gerstmann-Sträussler-Scheinker syndrome (GSS) |
20(p12-pter) |
PRNP |
prion protein |
Pro102Leu |
No |
|
Ataxia-telangiectasia-like disorder (ATLD) |
11q21 |
MRE11 |
part of the MRN repair complex |
? |
No |
|
Ataxia due to Coenzyme Q10 deficiency |
1q42.13 |
CABC1/ADCK3 |
kinase that modulates synthesis of CoQ10 |
multiple mutations |
No |
|
ARSACS (Charlevoix-Saguenay) |
13q12 |
SACS |
? |
6594delT, 5254C>T |
6594delT testing available via 23andme |
|
HSP7 |
16q24.3 |
SPG7 |
paraplegin required for m-AAA protease/ribosomal assembly |
multiple |
Yes |
|
Adult-onset Alexander disease |
17q21 |
GFAP |
function poorly understood |
multiple |
Yes |
|
Cerebro-tendinous xantho-matosis (CTX) |
2q33 |
CYP271A |
sterol oxidase |
multiple |
Yes |
|
CAPOS syndrome |
19q13.2 |
ATP1A3 |
Na+/K+ ATPase α3 |
c.2452G > A (p.(Glu818Lys)) |
Yes via Invitae |
Ataxia is most often due to dysfunction of the cerebellum or its inflow or outflow tracts. Acute lesions of the cerebellum are often severe but may improve gradually with a relatively good prognosis. Chronic degeneration of the cerebellum usually leads to gradual, persistent decline. Vestibulocerebellar lesions tend to result in impaired equilibrium and ataxic gait. Spinocerebellar lesions involve the vermis and tend to cause truncal ataxia and ataxic gait with minimal limb involvement. Neocerebellar lesions involve the cerebellar hemispheres and tend to result in severe limb ataxia. Less commonly, motor or sensory lesions can lead to a similar-appearing ataxic movement disorder (47; 46). Infectious, vascular, structural, and other causes of ataxia produce neurologic symptoms by mechanical disruption of the cerebellum or its numerous connecting pathways.
The dominant ataxias, spinocerebellar ataxia types 1, 2, 3, 6, 7, 12, and 17 and dentatorubropallidoluysian atrophy (DRPLA) share a common type of genetic mutation unique to neurodegenerative disorders called a cytosine-adenine-guanine repeat expansion. Within each of the responsible genes is a segment containing the trinucleotide cytosine-adenine-guanine (CAG), which encodes for the amino acid glutamine. The responsible mutation for each disorder is an increase in the number of CAG repeats leading to the production of normal amounts of an enlarged protein containing an excessive number of glutamine moieties. CAG repeat expansion does not appear to disrupt the translation of the gene or synthesis of the protein. The RAS-MAPK-MSK1 pathway has been implicated in modulating expression of ATXN1 protein in SCA1 (101). Diminished Nemo-like kinase (NLK) expression in SCA1 knock-in mice markedly improves phenotype, suggesting that NLK may play an important role in SCA1 pathogenesis (66).
Pathologically, synaptic loss is most severe in the olives in spinocerebellar ataxia type 1 and in the cerebellum and brainstem in spinocerebellar ataxia type 2; synaptic loss is much more variable in location and extent in spinocerebellar ataxia type 3 (36; 73). Work with transgenic mouse models of spinocerebellar ataxia type 1 has shown that the abnormal ataxin-1 protein is cleaved and forms inclusion bodies within the nucleus of the Purkinje cells that are destined to die. Preventing cleavage of the abnormal protein or its translocation into the nucleus of the cell appears to slow the expected course of the disease, whereas inhibiting the formation of inclusion bodies actually promotes the disease process (70). Analysis of spinocerebellar ataxia type 1 transgenic mice showed evidence of downregulation of several genes expressed by Purkinje cells, and it appears that several of these genes play a role in calcium regulation (80). Phosphorylation of ataxin-1 at serine 776 has been suggested as an important mechanism necessary for the development of degeneration in transgenic mice (34).
The pathogenesis of spinocerebellar ataxias that are not due to expanded CAG repeat remains unclear. Spinocerebellar ataxia type 10 is associated with an expanded adenine-thymine-thymine-cytosine-thymine (ATTCT) pentanucleotide repeat in intron 9 of the SCA10 gene (85). This is one of the largest expansions in the human genome, and evidence points to the role of expanded ATTCT tracts in unwinding DNA leading to instability (81). Point mutations in fibroblast growth factor 14 and protein kinase C gamma have been found in ataxia families without anticipation (18; 135). The identification of TGGAA pentanucleotide repeats on chromosome 16p22 in a large number of Japanese patients with spinocerebellar ataxia seems to be a more plausible pathogenetic mechanism than a single mutation in an untranslated region of the puratrophin-1 gene (PLEKHG4) (115).
Intronic expansion is also a feature of Friedreich ataxia caused by expanded guanine-adenine-adenine (GAA) repeat within the first exon of the frataxin gene (15). Rarely, some patients are found to be compound heterozygotes with a GAA triplet repeat expansion in one allele and a point mutation in the second allele of frataxin. Frataxin is localized to mitochondria and plays a role in the transport of iron out of the mitochondria, so it is thought that Friedreich ataxia may be a result of disrupted iron homeostasis. This theory is supported by analysis of frataxin-deficient mice as these animals exhibited time-dependent intramitochondrial iron accumulation and neurodegeneration (107). The number of GAA repeats is not as predictive as age of onset, with the latter more likely due to the combined result of several genetic and environmental factors (77).
De novo mutations in MORC2 have been described to cause early onset cerebellar atrophy together with spinal muscular atrophy like neuropathy and diaphragmatic paralysis. The test for this disorder is not available commercially (149). Regarding recessive ataxia, mutations in SLC52A2 gene for the riboflavin transporter have been linked to spinocerebellar ataxia with blindness and deafness type 2 (06).
The epidemiology of ataxia is difficult to generalize because it is dependent on the epidemiology of a large array of many disorders that result in ataxia. The epidemiology of the hereditary and sporadic degenerative ataxias is somewhat better studied (117). As a group, the ataxias have an overall prevalence of about 5 in every 100,000 individuals. Friedreich ataxia is the most common hereditary ataxia, most commonly having onset at less than 25 years of age, but occasionally presenting later. Friedreich ataxia is more common in individuals of French-Canadian or Acadian descent. This type of “founder effect” is described as the presence of an otherwise uncommon inherited disease gene in a progenitor of a physically or culturally isolated population, which then increases in frequency within that isolated population over succeeding generations (45; 99). In the United States, spinocerebellar ataxia type 2 and type 3 appear to be more common than spinocerebellar ataxia type 1, but it is unclear how common other types of spinocerebellar ataxias will prove to be. Most autosomal dominant ataxias begin during adult years, but due to the dynamic nature of the gene mutations responsible for these diseases, childhood onset of any of these syndromes is possible. Based on an extensive review of published data, the prevalence of childhood ataxia in Europe was estimated to be 26 out of 100,000 children and likely reflects a minimum prevalence worldwide (92).
The currently known dominant ataxia genes appear to account for between 25% and 75% of dominant ataxia families, depending on the population studied (38; 64; 91; 93; 100). A study of apparently sporadic cases of ataxia identified a known type of ataxia in 19% of affected subjects; Friedreich ataxia was the most common, followed by spinocerebellar ataxia type 6 and type 8 (118).
Epidemiologic studies focusing on sporadic cases of olivopontocerebellar atrophy found no association with head trauma, smoking, alcohol use, rural living, or well water. Affected individuals had less medication usage and a lower frequency of hypertension than unaffected individuals (71).
Prevention of ataxia requires prevention of the factors underlying the specific cause. Avoidance of chronic alcohol abuse can minimize the frequency of alcohol-induced ataxia. Diagnostic, predictive (testing of an adult who is at risk but who does not yet have symptoms), and prenatal testing is possible for adult-onset hereditary ataxias whose causative genes have been identified (see Table 2). For Friedreich ataxia, carrier testing of relatives of an affected person may be appropriate such that appropriate genetic counseling could be provided. Patients with Friedreich ataxia should be referred to a cardiologist and endocrinologist for monitoring and management of potential cardiac and diabetic complications of this disorder.
Prevention of ischemic stroke by reduction of known risk factors such as hypertension, tobacco use, and diabetes mellitus is likely to be beneficial in reducing the chances of developing ataxia from cerebrovascular disease. People known or suspected of having a hereditary form of ataxia should receive genetic counseling so they become aware of their risk of having affected children, the reproductive options available to them, and the potential risks of testing for the condition on their employability, insurability, and healthy relationships with family and friends.
The differential diagnosis of ataxia includes a large number of conditions. These are divided by category below; this list is not exclusive but is intended to highlight the more common conditions. The list of genetic conditions, alone, in which ataxia may be a symptom numbers more than 780.
|
Vascular | |
|
• Ischemic stroke (arterial or venous) | |
|
Trauma | |
|
• Injury to the bilateral posterior parietal cortex (Balint syndrome), cerebellum, spinal cord, or peripheral nerves | |
|
Developmental and Structural | |
|
• Dandy-Walker malformation | |
|
Neoplastic/paraneoplastic | |
|
• Astrocytoma | |
|
Infectious | |
|
• Viral: Epstein-Barr virus, varicella zoster, herpes, human immunodeficiency virus, hepatitis, subacute sclerosing panencephalitis, progressive multifocal leukoencephalopathy, rabies (pathologic though typically not clinical involvement of the cerebellum) | |
|
Inflammatory | |
|
• Postinfectious viral cerebellitis in children | |
|
Metabolic | |
|
• Leukodystrophies (eg, metachromatic leukodystrophy, adrenoleukodystrophy, Alexander disease, Krabbe disease, Pelizaeus-Merzbacher disease) | |
|
Toxic- or drug-related | |
|
• Medications (eg, phenytoin, carbamazepine, oxcarbazepine, lamotrigine, valproic acid, vigabatrin, gabapentin, pregabalin, tiagabine, cyclosporine, methotrexate, topiramate, amiodarone, cisplatin, lithium, barbiturates, fluorouracil, cytarabine, zolpidem, nitrous oxide) | |
|
Hereditary and sporadic ataxias (Table 1; Table 2) | |
|
• Friedreich ataxia | |
|
As a symptom of other neurologic disorders | |
|
• Acute neuropathies (Guillain-Barré syndrome) | |
|
As part of other genetic syndromes | |
|
• Fragile X syndrome (premutation carriers) | |
Careful attention to history, examination, and use of brain MRI can significantly shorten the long list of diagnostic possibilities in a patient with ataxia (104). Of the most commonly acquired causes of ataxia, tumors, structural and developmental anomalies, and multiple sclerosis all have characteristic MRI findings. Vascular lesions and traumatic injury frequently warrant further imaging with magnetic resonance angiography (MRA). Most of the hereditary or sporadic degenerative ataxias show atrophy of the cerebellum and sometimes the pons and olives corresponding with the clinical characterization of a pure cerebellar disorder or a more complex, multisystem degenerative disorder. Leukodystrophies usually show striking white matter abnormalities. Select patients may also be identified with MRI of the spinal cord. For example, the cerebellum usually appears normal in patients with Friedreich ataxia, but the cervical spinal cord may reveal atrophy. Alcohol-related chronic ataxia typically shows prominent atrophy of the cerebellar vermis out of proportion to any atrophy of the cerebellar hemispheres.
The history and physical examination should help substantially in the diagnosis of stroke syndromes, trauma-related or posttraumatic ataxia, ataxia related to other movement disorders or neurologic disorders, and ataxia related to certain syndromes. A history of exposure to toxins or metabolic stresses is important to consider. Unfortunately, no test is available that can clearly diagnose alcohol-related cerebellar degeneration (136). Patients with a history of heavy alcohol use in the distant past or modest ongoing use require that the physician look for other indicators of alcohol-related systemic or neurologic effects and consider other diagnoses before concluding that the problem is exclusively alcohol-related, especially given that alcohol use can lead to other potentially treatable nutritional deficiencies.
If MRI does not show diagnostic abnormalities, laboratory studies may be obtained to look for evidence of suspected conditions. A reasonable laboratory battery should include a complete blood count; electrolytes; serum vitamin B12 and vitamin E levels; levels of any known or suspected toxins (ie, phenytoin, carbon monoxide, or alcohol); a urine toxicologic screen, including drugs and heavy metals; liver function tests, including albumin level; a venous ammonia level; serum electrophoresis with immunofixation; and thyroid studies. In the proper context, a screen for antibodies associated with paraneoplastic cerebellar disturbance might be considered (122). Gluten sensitivity with high titers of antigliadin antibodies has been suggested as a cause of ataxia, even in patients without typical gastrointestinal symptoms seen in celiac sprue (144). Antigliadin antibodies have been documented in 41% of patients with sporadic ataxia (50). However, antigliadin antibodies can be found in a high proportion of patients with familial ataxia, and reports of improvement of neurologic symptoms after gluten-free diet are conflicting (21). Tissue transglutaminase levels are thought to be a more sensitive and specific diagnostic indicator of celiac sprue and have been shown to correlate with ataxic findings (51). Investigation into other treatable causes of ataxia in appropriate patients might include serum vitamin B1 or erythrocyte transketolase levels, a fasting lipid profile, red blood cell folate, serum pyruvate and lactate levels, creatine kinase, serum iron studies, antinuclear antibody and anti-SSA/SSB antibodies, plasma/urinary amino acid screen, HIV antibody test, ceruloplasmin, 24-hour urinary copper, and a paraneoplastic antibody panel. Abetalipoproteinemia may be detected via low serum levels of low-density lipoprotein, apolipoprotein, and triglycerides, with treatment focusing on dietary modification and treatment of associated vitamin deficiencies (108). Serum cholestanol and urinary bile alcohol levels are abnormal in cerebrotendinous xanthomatosis, which is treatable with oral deoxycholic acid (108).
When initial laboratory studies do not yield a specific or obviously treatable diagnosis in a patient with chronic ataxia, the possibility of a primary degenerative condition becomes more likely. Careful consideration of the family history is important at this juncture, and detailed characterization of the neurologic condition may help direct the diagnostic process. Neuropsychometric testing may disclose and characterize dementia, neurophysiologic studies can document the presence and nature of an associated neuropathy, and spinal cord imaging or evoked potential studies may reveal evidence of spinal cord atrophy or dysfunction. Male patients with cognitive decline and ataxia may be premutation carriers for fragile X syndrome (55 to 200 CGG repeats) (62). These patients frequently develop additional neurologic problems, including parkinsonism, postural tremor, and polyneuropathy. Positive family history of a grandchild with mental retardation is commonly present, and one or more female family members may have a history of premature ovarian failure. Increased signal on T2-weighted MRI in the area of the middle cerebellar peduncles has been suggested as a specific radiological marker for this condition.
For the patient with a positive family history of ataxia with a dominant inheritance pattern, analysis of the testable dominant ataxia genes should be considered. Most laboratories offer a panel of gene tests, but because the clinical features of the dominant ataxias overlap, it may not be possible to exclude or select a particular genetic diagnosis reliably based on the clinical examination and adjunctive testing alone.
The role of genetic testing in a patient with apparently sporadic degenerative ataxia remains uncertain. Studies typically show a low yield of positive results when patients are tested for dominant ataxia genes compared to a higher rate of positive results when testing for the frataxin gene (118). Although general approaches to treatment do not depend on a specific diagnosis, the genetic counseling of the patient and family are markedly altered by the diagnosis of a specific dominant or recessive genetic condition. Another potential benefit is the possibility of participating in clinical research trials that require a specific diagnosis prior to inclusion. Patients and involved family members should be made aware that some insurance companies or other third-party payers might not routinely cover the cost of all ataxic gene tests; however, obtaining prior authorization before ordering the studies in question likely reduces, but does not guarantee, the chances of denied coverage.
Treatments for most hereditary forms of ataxia are not readily available. Ataxia with vitamin E deficiency may respond to pharmacologic doses of vitamin E. Ataxia as a result of Wernicke encephalopathy usually improves with replacement of deficient thiamine (vitamin B1), although several months of recovery are often needed, and improvement in ataxia sometimes trails improved ophthalmoparesis. Sensory ataxia due to vitamin B12 deficiency often improves after replacement with vitamin B12 although resolution of the underlying neuropathy or associated cognitive deficits is often incomplete. A small study found that recombinant human erythropoietin injections three times a week increased frataxin levels, significantly improved ataxia on standard rating scales, and lowered oxidative stress levels, but four of the eight patients required phlebotomy due to elevated hematocrit, so further studies will be needed to evaluate benefit and the extent of blood monitoring (12).
Many of the symptoms associated with some forms of ataxia, such as spasticity, parkinsonism, sleep disturbance, muscle cramping, or depression, are amenable to drug therapy and are, therefore, important to recognize.
Management of ataxia includes identification and treatment of any condition underlying the specific symptom of ataxia. For degenerative ataxias, the next step is reaching a conclusion as to whether the condition is hereditary or sporadic and, if possible, identifying a specific genetic cause. Patients with a multisystemic disorder (eg, Friedreich ataxia and ataxia telangiectasia) should be referred to additional specialists when indicated, such as a cardiologist, endocrinologist, or clinical immunologist. Genetic counseling should begin early after the diagnosis of any hereditary ataxia. After the appropriate diagnosis is made, ongoing expectant management is important. Referrals to occupational therapy and physical therapy can help patients adapt to increasing disability. A social worker can be helpful as questions of permanent disability, home safety, or the need for long-term care placement arise. Family education about prognostic details and reviewing likely changes before they occur can help minimize unexpected surprises. Referral to appropriate support organizations, such as the National Ataxia Foundation, can be useful to patients and families with hereditary or sporadic ataxia. Pharmacologic management of related symptoms (ie, spasticity or sleep disturbance) can also improve quality of life. Establishing advanced care directives will help patients with progressive degenerative ataxia address uncertainty about whether their care would continue according to their wishes should they become unable to communicate effectively.
Deep brain stimulation has been studied in various patients with spinocerebellar ataxia with modest improvement on underlying tremor but no tangible benefit on ataxia observed (103; 123; 41). Bilateral deep brain stimulation of the ventralis intermedius nuclei has been investigated in a patient with fragile X tremor-ataxia syndrome with improvement noted in tremor but no significant change in ataxia (37). The use of deep brain stimulation in patients with ataxia remains investigational and is NOT an approved therapy by the Food and Drug Administration.
Alternative therapies such as herbal remedies, magnet therapy, acupuncture, and biofeedback are not known to improve ataxia. Many medications that act on the central nervous system, including anticonvulsants, can worsen ataxia. Some patients with parkinsonism or dystonia associated with spinocerebellar ataxia type 2 notice visible improvement after treatment with therapeutic doses of levodopa (84). A few anecdotal cases of improvement of ataxia symptoms following treatment with buspirone or N-acetylcysteine have been reported (82). Surgical treatments (transplantation of cerebellar allografts) have shown temporary benefits in an animal model but have not been studied in humans (67). Idebenone, a short-chain analogue of coenzyme Q10 is an effective free radical scavenger and has been used in Friedreich ataxia (39). First results of clinical trials demonstrated significant improvement of the cardiac function in these patients; however, there was no effect on a relentless progression of neurologic symptoms (13). Children can frequently tolerate higher doses of idebenone than originally studied, so further trials might be possible. One randomized trial showed evidence of a dose-dependent trend toward improvement of ataxia at higher doses of idebenone; however, no significant improvement was found (28; 27). There was no significant improvement of Friedreich ataxia on memantine (120). Refsum disease can be treated with plasma exchange, and cerebrotendinous xanthomatosis can be treated with oral deoxycholic acid (108). Alpha tocotrienol quinone is being investigated in Friedreich ataxia. This compound did not meet the primary end point in the placebo-controlled 6-month phase based on raw scores but disease progression at 24 months was improved compared to historical norms (150).
A novel approach for finding new treatments stems from targeting genetic networks that modulate expression of disease-causing genes. Park and colleagues found that suppressing the RAS-MAPK-MSK1 signaling pathway will reduce ATXN1 levels in SCA1 (101).
The transcriptome is an important therapeutic target. Yang and colleagues designed a molecule that binds to RNA AUUCU repeats present in SCA10 patient-derived fibroblast cells, leading to near wild-type level reduction in activation of caspase 3 (147).
Etravirine, a non-nucleoside reverse transcriptase inhibitor, has been shown to increase frataxin messenger RNA translation and normalize frataxin levels in cell culture models (02). Anti-ATXN3 microRNA can suppress the expression of ATXN-3 and reduce accumulation of mutant Ataxin-3 in a mouse model (111). CRISPR gene editing of the FMR1 gene has been attempted in induced pluripotent stem cells in fragile X-associated tremor/ataxia syndrome (148). Profound ethical questions need to be resolved before considering human genome editing as a treatment strategy.
None of the forms of ataxia have any direct effects on pregnancy. It is possible that an underlying condition responsible for ataxia can also independently affect pregnancy. Individuals with impairment of cardiac function, respiration, blood pressure regulation, or bowel, bladder, or sexual function as part of an ataxia syndrome may have difficulty during labor. Premature ovarian failure as a cause for infertility can often be seen in families with fragile X tremor-ataxia syndrome. Similarly, patients with vitamin E deficiency are often infertile. The vast majority of causes of ataxia do not have direct effects on fertility. Newborns with congenital malformations involving hypoplasia of the cerebellum and brainstem such as Joubert syndrome may be at risk for neonatal apnea due to disordered regulation of respiration, and neonatology consultations may be appropriate in these patients.
Ataxia is not known to have any effects on anesthesia. Some patients who receive nitrous oxide anesthesia develop subacute combined degeneration due to occult B12 deficiency. Neuropathic pain medications or other centrally acting medications used postoperatively could worsen ataxia, especially gait ataxia, so falling precautions should be introduced in appropriate patients. It is possible that an underlying metabolic disorder that causes ataxia could also have deleterious effects on the metabolism of anesthetic agents. One case report documented a patient with apparently altered electrophysiologic and autonomic responses to propofol that were not clinically significant (76). Congenital forms of ataxia, such as Joubert syndrome, that are associated with brainstem and other anatomical abnormalities may complicate the successful performance of an epidural block; the use of caffeine has been suggested to alleviate episodes of apnea in infants with this disorder (137). A patient with nonhereditary cerebellar degeneration has been described as successfully receiving neuromuscular blockade using vecuronium (97). Another case report described successful use of an epidural in a patient with spinocerebellar ataxia without acute or long-term neurologic deficits (112).
All contributors' financial relationships have been reviewed and mitigated to ensure that this and every other article is free from commercial bias.

Robert Fekete MD
Dr. Fekete of New York Medical College received consultation fees from Acadia Pharmaceutical, Acorda, Adamas/Supernus Pharmaceuticals, Amneal/Impax, Kyowa Kirin, Lundbeck Inc., Neurocrine Inc., and Teva Pharmaceutical, Inc.
See ProfileNearly 3,000 illustrations, including video clips of neurologic disorders.
Every article is reviewed by our esteemed Editorial Board for accuracy and currency.
Full spectrum of neurology in 1,200 comprehensive articles.
Listen to MedLink on the go with Audio versions of each article.
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Movement Disorders
Jan. 20, 2025
Movement Disorders
Dec. 29, 2024
Movement Disorders
Dec. 29, 2024
Movement Disorders
Dec. 29, 2024
Movement Disorders
Oct. 24, 2024
Neurogenetic Disorders
Oct. 23, 2024
Peripheral Neuropathies
Aug. 22, 2024
Movement Disorders
Aug. 22, 2024