General Neurology
Encephalitis lethargica
Dec. 28, 2024
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Worddefinition
At vero eos et accusamus et iusto odio dignissimos ducimus qui blanditiis praesentium voluptatum deleniti atque corrupti quos dolores et quas.
Toxoplasma gondii is a parasite that is present worldwide, infects multiple mammalian and avian species, and is a significant cause of asymptomatic and symptomatic human infection. The disorder is also capable of causing devastating prenatal neurologic injury. In adults, cerebral toxoplasmosis remains an important disorder in patients with HIV infection and AIDS, despite the advent of highly active antiretroviral therapy (HAART). Infection with Toxoplasma gondii is also an important issue in severely immunocompromised individuals who are not given prophylactic treatment to prevent infection by the organism. Less frequently, T gondii can cause central nervous system infection—including encephalitis—in apparently immunocompetent individuals. In AIDS patients, cerebral toxoplasmosis can coexist and be difficult to distinguish from cerebral cryptococcal infection and primary central nervous system lymphoma. Patients developing toxoplasma encephalitis have a high probability of early death. For this reason, antitoxoplasma prophylaxis should be maintained in HIV-infected patients who experience failure of antiretroviral therapy, and HAART should be initiated as soon as possible after toxoplasma encephalitis diagnosis. Prophylactic treatment should also be initiated in severely immunocompromises patients with other disorders.
|
• Toxoplasma gondii is a widely distributed protozoan that is found in multiple animal species. The definitive hosts, with regard to human infection, are felines. Human infection is common and may result in lifelong persistence of the organism in systemic tissues and/or within the central nervous system or eyes. | |
|
• Infection with Toxoplasma gondii in children or adults is most commonly acquired from ingestion of material contaminated with cat droppings or by eating undercooked meat containing toxoplasma cysts. Less frequently, the organism can be transmitted by blood transfusion or organ transplantation. | |
|
• T gondii modulates gene expression of brain endothelial cells to promote its own migration through the blood-brain barrier. | |
|
• Prior to the advent of the acquired immunodeficiency syndrome (AIDS), symptomatic invasion of the central nervous system in adults was unusual, most frequently occurring in the setting of immunosuppression. | |
|
• The advent of acquired immunodeficiency syndrome brought a marked increase in cerebral toxoplasmosis. Numbers of cases of cerebral toxoplasmosis in HIV infected individuals has fallen with the advent of highly active antiretroviral therapy (HAART). | |
|
• Prevention of congenital toxoplasmosis requires an active antenatal screening program, and prevention of toxoplasmosis in immunosuppressed individuals requires prophylactic antibiotic therapy. Seropositivity for toxoplasmosis of >200 IU/mL in serum is of great help in the antemortem diagnosis. | |
|
• The incidence of cerebral toxoplasmosis during pregnancy is low. |
Although Toxoplasma gondii was probably first identified in 1900 in Java sparrows by Laveran (63), the organism was given its current name following its recovery from the tissues of a hamster-like African mammal, Ctenodactylus gundi (29). Association of the agent with humans was first described in 1908 in Panama, where the organism was detected in muscle biopsies (26). However, for several decades thereafter, T gondii was confused with either Sarcosporidia or Encephalitozoon. The first case of congenital toxoplasmosis infection was described in 1923 (50). This patient had typical clinical features of congenital toxoplasmosis with unilateral micro-ophthalmia, hydrocephalus, and seizures. At postmortem, aqueductal stenosis and retinal lesions were present, and “sporocysts” were described in the retina. Sixteen years elapsed before toxoplasma was established as a causative agent for neurologic disorders in children, now termed “congenital toxoplasmosis,” in an extensive review by Wolf and colleagues (108).
Acquired toxoplasmosis was first described in 1940 in a Peruvian man who was immunosuppressed due to a preceding Bartonella infection (90). Although this patient had lymphadenopathy at autopsy, lymphadenopathy was not recognized as a characteristic sign of toxoplasmosis until 1951, when it was described during pregnancy (39).
Prior to the advent of AIDS, symptomatic central nervous system by T gondii was uncommon and was most often seen in the setting of immunosuppression (103). The first cases of CNS toxoplasmosis complicating HIV infection were described in 1983 (68), and the agent was rapidly recognized as a significant cause of CNS infections in HIV-infected patients (66). Toxoplasma chorioretinitis and cerebral toxoplasmosis have since been associated with treatment with anti-TNF-alpha agents (62), as well as with aggressive immunosuppression following hematopoietic stem cell or solid organ transplant (25; 72).
|
• Toxoplasma gondii is almost always acquired by ingestion; this may be from undercooked meat from infected animals or by eating food contaminated by cat droppings. Infection may rarely be acquired by blood transfusion or organ transplantation. | |
|
• Initial infection is usually asymptomatic but can result in a syndrome of fever, lymphadenopathy, and splenomegaly closely resembling infectious mononucleosis. | |
|
• Primary infection—even when asymptomatic—may be accompanied by a parasitemia, which can last for months. During infection, the organism can infect and persist in multiple tissues including the central nervous system and eyes. | |
|
• In adults, central nervous system infection is largely confined to immunocompromised patients and is the result of reactivated infection; this may result in cerebral abscesses, encephalitis, or meningitis. Retinal involvement can also occur, as, more rarely, can cases of toxoplasma myositis. | |
|
• Infection with T gondii in utero can result in devastating neurologic consequences. T gondii infection in children, however, is rare, even in the setting of immunosuppression. |
With the exception of rare individuals who are infected by the organism during blood transfusion (36), or by toxoplasma in donor tissue (89), T gondii is virtually always acquired by ingestion, either of infected, undercooked meat or ingestion of fruits or vegetables contaminated by cat droppings (51; 48; 31; 30). Humans may become infected with T gondii at any time during life. Seroprevalence rates increase with age, and are much higher in populations where ingestion of uncooked meat is common. Initial infection may be followed by prolonged parasitemia: in studies employing mouse inoculation, prior to the advent of AIDS, T gondii could be isolated from patient blood for as long as 14 months (77). The organism may invade–and remain latent in–multiple organs. In immunocompetent patients, primary T gondii infection is usually asymptomatic. However, some individuals may develop a mononucleosis-like syndrome characterized by lymphadenopathy, splenomegaly, and nonspecific symptoms of infection including low grade pyrexia, malaise, or myalgia (32; 47; 31).
Neurotoxoplasmosis and ocular toxoplasmosis are largely limited to immunocompromised individuals, in particular patients with HIV, and usually represents reactivation of previously dormant disease. Central nervous system involvement may take on several forms.
Cerebral abscesses. Toxoplasma cerebral abscesses occur most frequently in patients with inadequately treated HIV infection and/or failure of antiparasitic prophylaxis and has, over time, represented the most common cause of space-occupying lesions in AIDS patients (106; 31). Toxoplasma abscesses may also develop in individuals immunosuppressed for hematopoietic or stem cell transplantation or those receiving aggressive chemotherapy or immunosuppressive therapy for malignancy or autoimmune conditions (31). The abscess may be single; more commonly, multiple abscesses may be present (31). Clinical manifestations typically evolve over several weeks and may be accompanied by focal signs referable to the site of the abscess. T gondii infection has a predilection for the basal ganglia and may result in movement disorders, such as hemichorea, hemiballism, parkinsonism, or a rubral tremor (86; 84). In fact, to date all patients with AIDS and hemiballism or chorea have been proven to have cerebral toxoplasmosis (84).
Multiple cerebral abscesses are commonly present, which may result in multifocal symptoms including visual field deficits, focal seizures, aphasias, hemiparesis or hemisensory deficits, cranial nerve palsies, or cerebellar dysfunction. Nonfocal symptoms, such as a confusional state or personality disorder, may be an initial manifestation, but as the disease progresses focal symptoms eventually appear. Spinal cord involvement is uncommon (38).
Encephalitis. Less frequently, cerebral toxoplasmosis may result in encephalitis, which most commonly occurs in the setting of HIV infection or in transplant patients (40; 09; 95), and it may occur as an isolated condition or be accompanied by focal abscesses. Patients with encephalitic features develop varying degrees of altered levels of consciousness, generalized seizures, and headaches. The encephalitis may be accompanied by disseminated systemic disease, and the organism may invade the lymph nodes, heart, muscle, lung, or marrow. Female sex, severe immunodeficiency, and absence of primary toxoplasma encephalitis prophylaxis significantly increased the risk of toxoplasma encephalitis (40; 09; 95). In an Italian series, and previous exposure to antiretroviral therapy reduced the probability of disease occurrence (07). Pradhan and colleagues describe three distinct groups of toxoplasma meningoencephalitis in HIV seronegative patients: focal encephalitis, multifocal encephalitis, and diffuse meningoencephalitis (92). The groups had characteristic distinct symptoms and imaging features, with some overlap. The course of encephalitis is variable and may progress over several months to a more fulminant disease resulting in death within two weeks. In early stages, neuroimaging studies may be normal. Patients with AIDS who develop diffuse encephalitis typically have a subacute course of up to eight weeks. In this patient population, toxoplasmosis is usually limited to the brain. The cerebrospinal fluid shows a mild mononuclear pleocytosis with an elevated protein and normal or reduced glucose. Patients with underlying malignancies such as Hodgkin disease, depending on the degree of immune suppression, may also develop either abscess or diffuse or multifocal encephalitis with pathological findings of multifocal cysts (103). Abedalthagafi and colleagues have reported a 70-year-old male with stage IV chronic lymphocytic leukemia complicated by aplastic anemia, with a normal neurologic examination and imaging studies (02). But at autopsy, the brain revealed multifocal cysts and free tachyzoites of Toxoplasma gondii with diffuse microglial nodules and no necrosis.
Spinal cord involvement. Spinal cord involvement by T gondii is less common and usually occurs in significantly immunocompromised individuals. However, treatment-responsive spinal cord involvement due to T gondii has been reported in immunologically intact individuals (73).
Retinochoroiditis. Retinochoroiditis due to toxoplasmosis is usually seen in infants with congenital toxoplasmosis but may also occur in immunosuppressed adults, including individuals with poorly controlled HIV infection or stem cell or solid organ transplantation (45; 88; 42; 54). For unexplained reasons, in HIV-positive patients ocular toxoplasmosis is a much less common opportunistic infection than is cytomegalovirus retinitis. Unlike cytomegalovirus retinitis, however, toxoplasmosis can cause a progressive intraocular infection, panophthalmitis, and orbital cellulitis (79). Retinochoroiditis is the most common ocular manifestation of ocular toxoplasmosis (54). Lesions can be single or multiple, and active lesions usually present as grey-white foci of retinal necrosis with adjacent choroiditis, vasculitis, hemorrhage, and vitreitis, frequently involving the posterior pole with secondary spreading to involve the vitreous and choroid (04; 08). The retina is edematous with small hemorrhages and exudates. Healed lesions are densely pigmented with irregular borders and central atrophy. Frequently, multiple lesions at various stages of inflammation and healing are seen. Retinal involvement is frequently accompanied by anterior uveitis.
Extracerebral sites may be involved with or without toxoplasma encephalitis in patients with AIDS. Eza and Lucas report a case of fulminant myocarditis and another with fatal pneumonitis and renal failure (34). Toxoplasma pneumonitis is responsible for less than 1% of HIV-related pulmonary complications. Invasion of muscle may produce symptomatic myositis; this may occur in patients with AIDS but may also occur in the other immunocompromised states or in the absence of identified immune deficiency (41; 78; 91; 24).
Congenital toxoplasmosis. T gondii can cross the placenta to cause devastating infection in the fetus or neonate (58; 64; 74). Brains of affected infants may show multiple nodular foci of hematogenous dissemination with the formation of a focal inflammatory reaction. This is often seen in the periventricular and subpial regions with slight perilesional edema. The severity of the disease in the fetus is dependent on gestational age at time of infection. Clinically significant abnormalities often occur before 26 weeks’ gestation with highest risk at 10 to 24 weeks (58). The clinical manifestations are variable, although the classical triad is hydrocephalus, chorioretinitis, and multifocal cerebral calcifications. In neonates with hydrocephalus, stenoses of the aqueduct may be due to edema rather than permanent scarring; therefore, if treated, the clinical manifestations may resolve. Mild cases may go unrecognized or may have isolated chorioretinal scars (97; 57; 58), but the infection may result in microcephaly. In contrast to congenital toxoplasmosis, CNS toxoplasmosis is rare after the neonatal period and is rare even in immunocompromised children.
Neurologic disorders are a common reason for intensive care unit admission in AIDS patients, and the mortality rate of untreated HIV-infected patients admitted to the intensive care unit for CNS disorders is 25% to 50% (80; 82; 59). Toxoplasma CNS infection has been the most common cause of focal neurologic disease in patients with AIDS (07; 106). Antibiotic therapy is highly effective in reversing the symptoms of cerebral toxoplasmosis and controlling the infection. Untreated, however, cerebral toxoplasmosis results in progressive multifocal deficits leading to cerebral herniation and death. Factors that determine poor outcome in disseminated toxoplasmosis, including toxoplasma encephalitis, are advanced stage of underlying immunodeficiency, cytotoxic chemotherapy, steroid therapy, splenectomy, concurrent infections, and radiation therapy. Outcome is improved by instituting appropriate therapy early in the disease course.
A 26-year-old woman with HIV infection and a history of intravenous heroin abuse developed sudden violent movements of the right hand and leg. She was unable to dial a telephone or write. She also had left-sided headaches. She had mild spontaneous grimacing of the right side of her face and continuous, nonpatterned, violent, coarse jerking, choreic-ballistic movements of the right arm and leg that were so intense that while standing she would lose her balance. CT of the brain showed a lucency in the left basal ganglia and left frontal region with contrast enhancement.
Cerebrospinal fluid showed three mononuclear white blood cells, protein of 38 mg/dL, and glucose of 55 mg/dL. The CSF IgG titer for toxoplasmosis was 1 to 160. This patient was treated with sulfadiazine and pyrimethamine along with haloperidol to control the movements. Complete resolution of the cerebral abscess was noted two weeks later; however, mild choreiform movements persisted in the right arm (85).
Comment. This case illustrates that cerebral toxoplasmosis must be suspected if a patient with HIV infection presents with hemichorea or ballism, and that empirical therapy with antitoxoplasma medication should be initiated. Even though the cerebral abscess may respond dramatically to antibiotics, the movement disorder may persist and require pharmacological intervention.
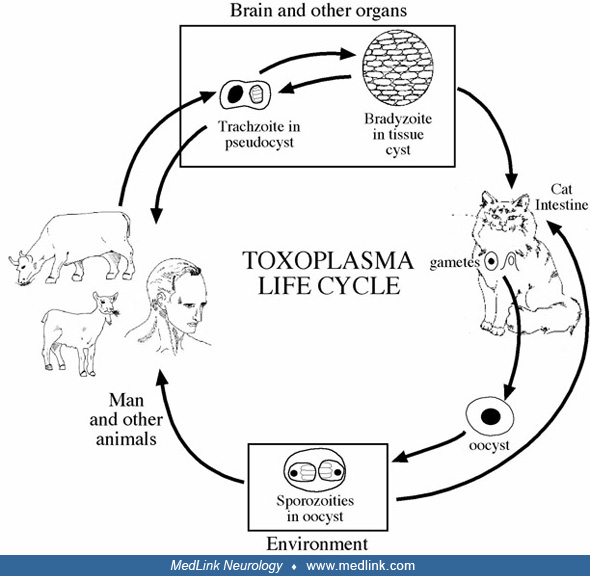
The causative agent of toxoplasmosis is a coccidian parasite, Toxoplasma gondii, so named because the organism was first identified in the African rodent Ctenodactylus gundi. The word toxoplasma was derived from the Greek word “toxon” or “arc.” However, the definitive host for the toxoplasma is the cat family. Toxoplasma exists as oocysts, bradyzoites, and tachyzoites. Oocysts are found only in cats, and this form is infective only when completely sporulated. Thus, humans and animals almost always acquire the infection by eating animals with disseminated infection or by ingesting oocysts shed in cat feces (49; 43; 99; 31).
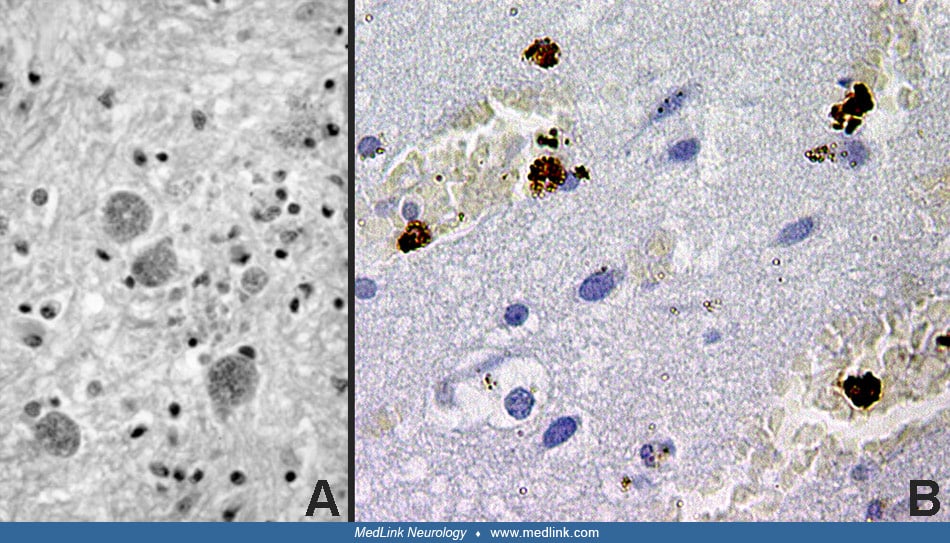
T gondii is an obligate intracellular protozoan. Oval or crescent shaped tachyzoites are typical of the acute infestation, and within parasitized cells they are usually clustered within vacuoles. The cytoplasm of parasitized cells can be burdened with rapidly multiplying organisms, which may be released when the cell ruptures. The bradyzoite is characteristic of the chronic stage of the disease and is contained within cysts. These cysts most commonly occur in the CNS and cardiac and skeletal muscles.
They vary from approximately 15 µm, which contain just a few bradyzoites, up to 100 µm, which contain thousands of bradyzoites. The bradyzoites replicate slowly, and the cysts can persist for months and years and may elicit little tissue reaction. The life cycle of toxoplasma is reviewed by Evans (33).
Yong and colleagues reported a case of cerebral toxoplasmosis in a previously healthy 41-year-old man who was found to have a genetic defect in CD40 ligand, resulting in the X-linked hyper-IgM syndrome despite normal surface protein expression on flow cytometry (110). This suggests that primary immunodeficiencies can first present late in life with a relatively mild phenotype, and a normal protein expression does not necessarily rule out hypomorphic mutations.
Both cellular and humoral immune responses are critical in controlling the proliferation of the parasites by inducing lysis of the organism or enhancing cyst formation in tissues. Resistance against cerebral toxoplasmosis is mainly sustained by interferon-[gamma]-dependent, CD8+ T cell-mediated immunity (31). For this reason, T gondii affects the brain almost exclusively in patients with compromised cellular immunity (102). Deficiency of IgA, IgG, and IgM, referred to as common variable immunodeficiency, is the most frequent of the primary specific immunodeficiencies (01). Cerebral toxoplasmosis might be facilitated by preexisting common variable immunodeficiency. In cases of unexplained cerebral toxoplasmosis, screening for humoral immunodeficiencies may be warranted.
Experimental data clearly support the role of CD4 cells and gamma interferon in preventing the proliferation of brain cysts (102; 31). Hence, in the setting of immunosuppression and in the case of patients with HIV infection, when CD4 T-lymphocyte count falls below a critical level, toxoplasma is reactivated. Depending on the degree of immune response that the host is able to mount, clinical manifestations may result. For example, if the organisms can be walled off, an abscess may form; a lack of this may result in disseminated encephalitis with diffuse invasion of the organism in the brain. Intermediary stages may result in nodular lesions. Systemic dissemination of the organism may result in embolic infarcts within the brain, resulting in a hemorrhagic infarct.
Lachenmaier and colleagues report that “T. gondii modulates gene expression of brain endothelial cells to promote its own migration through the blood-brain barrier in a 'Trojan horse' manner, and cells expressing CD11b either with or without CD11c are likely candidate cells for the intracellular transport of T. gondii across the BBB,” (61). Upon infection of humans and animals with Toxoplasma gondii, the parasites persist as intraneuronal cysts that are controlled, but not eliminated by the immune system. There is a fine-tuned balance between the parasite, the brain, and the immune system (14). In particular, intracerebral T cells are crucial in the control of T gondii infection and are supported by essential functions from other leukocyte populations. Additionally, brain-resident cells including astrocytes, microglia, and neurons contribute to the intracerebral immune response by the production of cytokines, chemokines, and expression of immunoregulatory cell surface molecules, such as major histocompatibility (MHC) antigens.
It is known that cerebral infection with the parasite Toxoplasma gondii is followed by activation of resident cells and recruitment of immune cells from the periphery to the CNS. Biswas and colleagues showed that a subset of myeloid cells, namely Ly6C(high)CCR2(+) inflammatory monocytes that infiltrate the brain upon chronic T gondii infection, plays a decisive role in host defense (13). They further showed that the recruitment of Ly6C(high) monocytes to the CNS is regulated by P-selectin glycoprotein ligand-1. Depletion of this monocyte subset resulted in elevated parasite load and decreased survival of infected mice, suggesting its crucial role in parasite control and immune regulation of the CNS.
Although cats serve as natural reservoirs of toxoplasma, virtually any animal – including birds – that ingests material contaminated with oocysts can get infected (99). The frequency and prevalence of toxoplasma infection in humans varies considerably depending on age, dietary habits, climate, and proximity to cats. Congenital infection nearly always occurs with primary maternal infection during gestation, with an estimated incidence of 6 per 1000 pregnancies in the United States. The risk for pregnant women to acquire acute infection is directly related to the amount of exposure (eg, ingestion of raw or undercooked meat or of oocysts) and to the likelihood that she has antibodies to T gondii (57). Acquired toxoplasmosis is a common infection after birth. Older data suggested that IgG antibodies to T gondii could be detected in 50% of individuals in the United States (97), whereas in parts of Western Europe and Central America it can be as high as 70% to 90%. Data from the United States provide an overall IgG seroprevalence rate of 11.4%, with somewhat higher rates of seropositivity in males, in non-Caucasian individuals, and in individuals living below the poverty line or born outside the United States (52). Only approximately 10% of acutely infected individuals (normal hosts) have clinical signs and symptoms, which are often mild, but in marked contrast to these patients, T gondii may cause devastating and often fatal infection (encephalitis, myocarditis, hepatitis, pneumonitis, and disseminated infection) in immunocompromised patients. Cerebral toxoplasmosis, although common in the early years of the AIDS epidemic, has been gradually declining as the use of prophylactic drug therapy and antiretroviral agents has increased (10; 03). In contrast, the rate of T gondii infection in aggressively immunosuppressed transplant patients has been rising (98). Busemann and colleagues report three cases of toxoplasmosis identified among 155 allogenic stem cell transplantations (16). The mortality rate was high and all three patients died. Based on their experience, the authors recommend a real-time PCR targeting a 529-bp genomic fragment, for diagnosis over the conventional T gondii PCR. From the PcP prophylaxis point of view, they further encouraged the use of trimethoprim-sulphamethoxazole over pentamidine.
|
• No vaccine is currently available to prevent toxoplasma infection. | |
|
• HIV-infected patients should be tested for toxoplasma antibodies soon after diagnosis. Similar consideration should be given to individuals undergoing aggressive immunosuppression for organ or stem cell transplantation. Seropositive patients not receiving prophylaxis for pneumocystis pneumoniae should be treated with sulfadiazine, pyrimethamine, and folinic acid. | |
|
• Patients at risk should be advised to avoid eating undercooked meat or unwashed fruits and vegetables. Patients owning (or caring for) cats should use meticulous hygiene in dealing with the animals and their litter boxes. | |
|
• Sulfadiazine/pyrimethamine and spiramycin have both been used to prevent congenital toxoplasma infection. |
No effective vaccine is currently available, and once the parasite is encysted, it is resistant to antibiotics. Hence, other steps are necessary for prevention of infection (55).
HIV-infected persons should be tested for IgG antibody to toxoplasma soon after the diagnosis of HIV infection to detect latent infection. All HIV-infected persons should be advised not to eat raw or undercooked meat. Meat should be cooked to an internal temperature of 150°F (66°C); meat cooked until it is no longer pink inside generally has an internal temperature of 165°F (74°C). Milk should be either pasteurized or boiled. HIV-infected persons should wash their hands after contact with raw meat and after gardening or other contact with soil. They should wash fruits and vegetables before eating them raw. Cat litter boxes should be changed daily, preferably by an HIV-negative, nonpregnant person; alternatively, the patient should wear gloves or wash hands thoroughly after changing the litter box. Patients should be encouraged to keep their cats inside and not to adopt or handle stray cats. Cats should be fed only canned or dried commercial food or well-cooked table food, not raw or undercooked meats. Patients need not be advised to part with their cats or to have their cats tested for toxoplasmosis.
Prevention of congenital toxoplasmosis requires an active antenatal screening program, and prevention of toxoplasmosis in immunosuppressed individuals requires prophylactic antibiotic therapy, most commonly employing spiramycin or pyrimethamine together with a sulphonamide (94; 15; 93). Toxoplasma-seropositive patients with a CD4+ T-cell count less than 100/μL should be administered prophylactic antibiotics to prevent reactivation of toxoplasmosis (94). The doses of trimethoprim and sulfamethoxazole recommended for pneumocystis pneumonia prophylaxis are effective against toxoplasmosis as well. If patients cannot tolerate trimethoprim and sulfamethoxazole regimens, dapsone plus pyrimethamine provide protection against toxoplasmosis. Prophylactic monotherapy with dapsone, pyrimethamine, azithromycin, clarithromycin, or atovaquone cannot be recommended on the basis of current data. Aerosolized pentamidine does not afford protection against toxoplasmosis.
Toxoplasma-seronegative persons not on a regimen of treatment for Pneumocystis pneumonia prophylaxis, which is also active against T gondii should be retested for IgG antibody to toxoplasma when their CD4+ T-lymphocyte count is less than 100/μL. Patients who have seroconverted should be administered prophylaxis for toxoplasmosis.
Patients with HIV or under chronic immunosuppression who have had toxoplasmosis should similarly be given prophylactic therapy against toxoplasma to prevent relapse. The combination of pyrimethamine plus sulfadiazine and folinic acid (leukovorin) is highly effective for this purpose. Pyrimethamine is a folate antagonist; leukovorin (folinic acid) can be converted to folic acid in humans, but T gondii organisms are unable to do so. Patients unable to tolerate sulfa drugs can be given pyrimethamine plus clindamycin; however, only the combination of pyrimethamine plus sulfadiazine appears to provide protection against Pneumocystis pneumonia as well. Pyrimethamine is a folic acid antagonist. Folinic acid, which is converted by humans to folic acid but cannot be converted by T gondii, is used to prevent folic acid depletion in the treated patient. Studies show that both primary and secondary prophylactic therapies can be safely discontinued in patients once the CD4+ T-lymphocyte count can be sustained at a level of greater than 100/μL and for longer than three months (81; 101). Mortality is higher in antiretroviral naive immigrants with neuro-AIDS treated on a neurologic intensive care unit, necessitating the need for greater availability of highly active anti-retroviral therapy worldwide to improve the outcome (17).
Cerebral toxoplasmosis remains a highly prevalent disorder of the CNS, even in the ART era, particularly among severely immunosuppressed patients and in the absence of prophylaxis (07). In children, incidence of cerebral toxoplasmosis is rare even in immunocompromised individuals. In patients who are seronegative for toxoplasmosis or are on prophylactic therapy for toxoplasmosis, but have positive imaging studies, other diagnoses should be considered, including tuberculosis, cryptococcosis, and fungal abscess. For an immunosuppressed patient who presents with focal neurologic signs and multiple cerebral ring-enhancing lesions on neuroimaging, cerebral toxoplasmosis would be the most likely diagnosis. In fact, this presentation is so characteristic that current guidelines suggest that all such patients be treated with antitoxoplasma medications (106). A lack of response to such therapy should alert the clinician about the possibility of other conditions, such as CNS lymphoma or progressive multifocal leukoencephalopathy. Rarely, cerebral infarcts, varicella zoster infection, and other bacterial, fungal, or parasitic infections may mimic such a clinical presentation. In patients with solitary lesions, the possibility of cerebral lymphoma would be more likely. Cerebral toxoplasmosis is common in advanced HIV disease. Primary cerebral lymphoma can coexist with cerebral toxoplasmosis, for over 30% of cases of primary lymphoma are associated with HIV infection (76). Although tissue diagnosis is the gold standard, MRI and PET imaging might help in differentiating the two conditions.
As noted, neurotoxoplasmosis in adults is closely associated with HIV infection or with aggressive immunosuppression. Neurotoxoplasmosis in nonimmunocompromised patients is rare.
The definitive diagnosis of toxoplasma encephalitis requires demonstration of tachyzoites in a biopsy specimen of the brain. This is infrequently done, however, and diagnosis involves clinical suspicion, imaging studies, serology, and PCR.
The presence of IgM antibodies in serum indicates a recent infection, and IgG shows previous infection. Rising IgG titers would be indicative of reactivation of toxoplasmosis. However, in the presence of severe immunosuppression, T gondii may reactivate with minimal changes in antibody titers (28). Chaudhry and colleagues shed light on reasons why routine screening for Toxoplasma gondii infection during pregnancy is not generally available (21). In their opinion, the reasons include low prevalence of the infection, high cost associated with testing, low sensitivity of screening tests, false-positive test results, and limitations of treatment effectiveness.
Hence, other diagnostic techniques may be necessary. Polymerase chain reaction testing on blood or buffy coat would demonstrate the presence of circulatory organisms. However, parasitemia may be present without encephalitis. Polymerase chain reaction testing on cerebrospinal fluid has 100% specificity for documented or presumed encephalitis with a sensitivity of about 90% depending on the primers and partial regression coefficient conditions used. Thus, polymerase chain reaction testing on cerebrospinal fluid is a useful diagnostic tool, if a positive result occurs (22; 53). Based on their study, Olariu and colleagues suggest that in infants with clinical and serologic findings suggestive of congenital toxoplasmosis and born to untreated mothers, cerebrospinal fluid polymerase chain reaction (CSF PCR) has the potential to increase the frequency of cases in which the diagnosis is confirmed (87). The emerging technique of metagenomic next generation sequencing may provide additional diagnostic capability in cases where toxoplasma encephalitis is suspected (46; 111; 69).
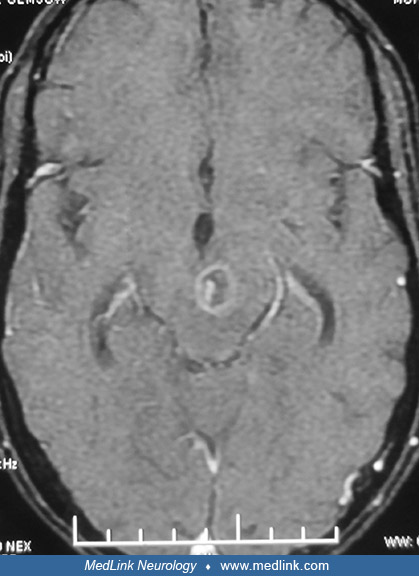
Neurotoxoplasmosis lesions are often seen as diffuse, low-signal intensity lesions on the unenhanced CT, and following intravenous contrast administration appear as highly variable heterogeneous enhancement. In MRI, these lesions will have low attenuation on T1 and increased signal on T2, often showing varying degrees of contrast enhancement and extensive perilesional edema. Neuroimaging techniques demonstrate the abscesses usually as multiple ring-enhancing lesions with mass effect and surrounding edema. They may be seen in the corticomedullary junction, and in patients with AIDS the lesions frequent the basal ganglia (84). MRI is more sensitive than CT in demonstrating these lesions; T2-weighted hyperintensity correlates with necrotizing encephalitis, T2-weighted isointensity with organizing abscesses (18). Nearly 40% of patients with CNS lymphoma may also have multifocal ring-enhancing lesions, although the lymphomas rarely invade the basal ganglia. Rarely, cerebral toxoplasmosis presents with nodular or nonenhancing lesions. In such patients with solitary brain lesions, cerebral lymphoma or other causes of mass lesions should be considered. Also, rarely cerebral toxoplasmosis may present with hemorrhagic lesions. In fact the combination of abscess and hemorrhage on neuroimaging should alert to the possibility of cerebral toxoplasmosis.
Cerebral toxoplasmosis and lymphoma are the most common focal destructive brain lesions in AIDS and can occur in isolation or together. A major consideration AIDS patients found to have ring-like lesions on MRI is thus determining whether these lesions represent toxoplasmosis or lymphoma. This can be challenging because lesions caused by these conditions may be indistinguishable on MRI. Helpful criteria based on imaging studies to distinguish toxoplasmosis from CNS lymphoma include subcortical location of toxoplasmosis lesions, their multiplicity, and the observation that enhancing wall of the lesions thinner than that observed in lymphomas. Two additional imaging findings may be useful in differentiating toxoplasma abscesses from lesions caused by lymphoma. The “eccentric target sign,” seen subcortically on T1 weighted MRI with gadolinium enhancement, consists of an annular enhanced area with an eccentric nodule along its wall (105). Although this lesion is seen in only 25% to 30% of patients, it is considered to be suggestive of cerebral toxoplasmosis with 95% specificity. Autopsy studies by Kumar and colleagues have demonstrated that the eccentric target sign noted on MRI corresponded histologically to a large necrotizing abscess containing concentrically thickened vessels (60). A second clue to diagnosis is the “concentric target sign" (70). This finding is observed in deep parenchymal lesions on T2-weighted images and consists of concentric alternating zones of hypo- and hyperintensities. It is believed to be more specific than and distinct from surface-based "eccentric target sign" in the diagnosis of cerebral toxoplasmosis and more useful in differentiation from other focal brain lesions in the context of AIDS. Based on the postmortem findings of a 40-year-old man with AIDS-associated cerebral toxoplasmosis, Mahadevan and colleagues correlated MRI findings of concentric alternating zones of hypo-, hyper-, and iso-intensities to zones of hemorrhage/fibrin-rich necrosis with edema, coagulative compact necrosis, and inflammation with foamy histiocytes admixed with hemorrhage forming the outermost zone, respectively (70). Leptomeningeal involvement is rare in cerebral toxoplasmosis unless it is secondary to a concomitant condition as a result of immunocompromised immune status. Both ventriculitis and corpus callosum involvement have been reported in toxoplasmosis but are uncommon (67).
A major concern in HIV patients with parenchymal brain lesions is whether the lesions represent toxoplasmosis or CNS lymphoma. Diffusion-weighted imaging and ADC correlation have been used to differentiate the two conditions, but considerable overlap occurs (100). Thallium-201 is readily taken up by lymphomas, and thallium-201 labeled SPECT has been used to differentiate the two conditions as has 18F-FDG PET/CT (71).
Nearly 90% of patients with cerebral toxoplasmosis will respond clinically and radiologically to drug therapy for cerebral toxoplasmosis by 14 days of treatment. Response to empirical therapy may, thus, be used diagnostically. Hemorrhagic conversion of the lesions often occurs during specific drug therapy for the condition, though it could happen before treatment as well. Treatment of CNS toxoplasmosis may also be complicated by immune reconstitution inflammatory syndrome (96).
In patients who present with movement disorders with cerebral toxoplasmosis, radiological improvement may occur despite no apparent improvement in the movement disorder. In such patients, it is essential to repeat MRI scans after 14 days of therapy to assess response to treatment (84). If corticosteroids are used, apparent clinical and radiological improvement may occur due to nonspecific, anti-inflammatory effects. In patients who fail drug therapy for toxoplasmosis as well as in some patients with solitary lesions, biopsy of the lesion may be necessary to establish the diagnosis by the demonstration of tachyzoites.
Adurthi and colleagues emphasize that seropositivity for toxoplasmosis of >200 IU/mL in serum is of great help in antemortem diagnosis, with the appropriate clinical setting, especially when a spinal tap is contraindicated, and a PCR is not available (05). Excretory-secretory antigens (ESA) or immunoblotting of CSF samples or both can add supportive evidence for cerebral toxoplasmosis in the presence of clinical, serologic and radiologic evidence suspicious of cerebral toxoplasmosis, especially in the latent phase (75).
Utsuki and colleagues report a case of primary CNS lymphoma in AIDS mimicking cerebral toxoplasmosis on MRI findings (104). Focal flavivirus infections, toxoplasmosis, and primary CNS lymphoma may mimic each other on their MRI findings, and the correct neuroimaging diagnosis can be made only by taking all relevant clinical and laboratory information into account, in addition to judicious use of confirmatory neuroimaging investigations, especially MR diffusion-weighted imaging, MR angiography, MR venography, and MR spectroscopy when needed (44). Oftentimes a tissue biopsy is needed to reach a definite diagnosis.
In HIV-infected patients, MRI cannot reliably differentiate between CNS lymphoma and nonmalignant CNS lesions, particularly cerebral toxoplasmosis. In their study on the utility of FDG PET-CT and MR spectroscopy in differentiating between cerebral lymphoma and nonmalignant CNS lesions in HIV-infected patients, Westwood and colleagues found that FDG PET-CT correctly identified all cases of CNS lymphoma and cerebral toxoplasmosis, supporting its use in this situation (107). They also found that MR spectroscopy was unhelpful in this clinical situation.
|
• The current standard of care is to treat presumptively for toxoplasma encephalitis when a characteristic neuroradiographic abnormality is seen on CT or MRI, and resort to brain biopsy only if improvement is not noted in 2 weeks (27; 25). | |
|
• The choice of drugs for treating cerebral toxoplasmosis is limited (37). Only three drugs are available, and of these, pyrimethamine and sulfonamide are invariably used in combination. Clindamycin is an alternative choice. | |
|
• Because pyrimethamine is a folate antagonist, patients receiving pyrimethamine should be treated with folinic acid (leukovorin), which humans can convert into folic acid but toxoplasma organisms cannot. | |
|
• Spiramycin, although effective against toxoplasma, has poor CNS penetration but achieves high concentrations in the placenta and is useful for treatment of toxoplasmosis during pregnancy. | |
|
• Because long-term maintenance therapy is a common practice, particularly in patients with AIDS, a wider choice of antibiotics is urgently necessary due to potential problems with drug resistance and side effects. See Table 1 for dosages of medications. |
|
Non-AIDS patients | ||
|
Pyrimethamine 100 mg twice on the first day, then 25 mg daily | ||
|
• Treat 1 to 2 weeks beyond resolution of clinical manifestations | ||
|
AIDS patients | ||
|
Pyrimethamine 200 mg on the first day, then 75 mg to 100 mg daily | ||
|
• Treat 1 to 2 weeks beyond resolution of clinical manifestations | ||
|
Pyrimethamine 25 to 50 mg daily | ||
|
• In case of a sulfadiazine allergy, substitute clindamycin 600 to 1200 mg intravenously four times daily for initial therapy, 450 to 600 mg orally four times daily for maintenance | ||
Sulfadiazine. Sulfadiazine is the sulfonamide of choice. It is highly protein bound with good penetration into the cerebrospinal fluid and other tissues. Sulfonamides are analogues of para-aminobenzoic acid, which is an essential precursor for the synthesis of folic acid. Sulfonamide, thus, competitively inhibits de novo synthesis in microorganisms that are obliged to make their own folic acid. The end result is an inhibition of nucleic acid synthesis. It limits replication rather than killing the organism. Common side effects include hypersensitivity, especially with prolonged treatment. The drug acts as a hapten, and on binding with proteins it produces an immune response, resulting in a rash. This complication is more frequently observed in HIV-infected patients than in other populations. Other immunological complications include lupus, polyarteritis nodosa, and myocarditis. This drug may cause hemolysis in neonates, resulting in kernicterus; however, this concern is overridden in treatment of proven congenital toxoplasmosis. In one study group, the outcome seen with sulfadoxine-pyrimethamine as compared to standard recommended treatment yielded comparable results. Tolerance to treatment was better and the adverse effects warranting change in treatment were fewer despite that only a few patients received folinic acid supplements (06).
Pyrimethamine. This drug selectively inhibits the microbial enzyme dihydrofolate reductase but does not have any effect on the human counterpart, which is structurally dissimilar. It acts synergistically with sulfadiazine on toxoplasma, producing 10-fold greater activity than that expected from addition of each drug together. Pyrimethamine is well absorbed and has a variable half-life from 1 to 4 days. The CSF levels of the drug are about one fifth of the serum levels. Folinic acid is administered concomitantly to prevent bone marrow toxicity induced by pyrimethamine (11). The frequency of side effects in patients with AIDS is 50%, and although many of these patients have to discontinue treatment, desensitization regimens have been effective in some (23).
Clindamycin. This drug binds to ribosomes and inhibits protein synthesis. The reasons for its selective toxicity in parasites are not fully understood. Clindamycin is highly lipid soluble and highly protein bound. It has good penetration into the eye and dense tissues, such as bone. Its penetration into cerebrospinal fluid is poor. It is mainly used in treatment of ocular toxoplasmosis and as a second-line drug for treatment of CNS toxoplasmosis (56). The most common side effect is diarrhea due to suppression of normal flora and proliferation of Clostridium difficile. If symptoms persist after discontinuing clindamycin, treatment with metronidazole or oral vancomycin may be necessary to control the clostridia.
Azithromycin. Experimental data have indicated that azithromycin is effective against the cyst form of parasite (20). The antitoxoplasmic effect of azithromycin and its synergistic activity with pyrimethamine or sulfadiazine have been demonstrated in several experimental models (19). Oral therapy with pyrimethamine and clarithromycin is effective for the treatment of acute toxoplasma encephalitis (35); and some have reported the occasional use of azithromycin in AIDS patients with toxoplasmic disease (109). In a patient with neurotoxoplasmosis who relapsed during conventional chemotherapy, association with azithromycin produced complete remission (83); azithromycin has been used in combination with sulfamethoxazole with good outcome in an immunocompromised child (111).
Toxoplasma encephalitis remains a highly prevalent disorder of the CNS, even in the ART era, particularly among severely immunosuppressed patients and in absence of prophylaxis. Considering that persons with toxoplasma encephalitis have a high probability of early death, prophylaxis should be maintained in immunosuppressed patients who experience failure of antiretroviral therapy, and HAART should be initiated as soon as possible after toxoplasma encephalitis diagnosis (07). Discontinuation of maintenance therapy against toxoplasmic encephalitis was generally safe, but may fail in a minority of patients. Patients who remain clinically and radiologically free of relapse at six months after discontinuation are unlikely to experience a relapse of toxoplasmic encephalitis (12). Lejeune and colleagues claim that combined antiretroviral therapy restores T gondii-specific CD4 T cell responses in most patients with AIDS who had previous toxoplasmic encephalitis (65). In addition, their data support the safety of withdrawing toxoplasmic encephalitis prophylaxis when the CD4(+) T-cell count returns to levels greater than 200 cells/μL.
The efficacy of prophylactic treatment of the mother to prevent fetal infection is uncertain and its use controversial. Because of the low incidence of cerebral toxoplasmosis during pregnancy and the possible teratogenicity associated with pyrimethamine treatment, chemoprophylaxis with pyrimethamine-containing regimens is not usually used but could be considered in mothers who seroconvert during pregnancy.
All contributors' financial relationships have been reviewed and mitigated to ensure that this and every other article is free from commercial bias.

John E Greenlee MD
Dr. Greenlee of the University of Utah School of Medicine has no relevant financial relationships to disclose.
See ProfileNearly 3,000 illustrations, including video clips of neurologic disorders.
Every article is reviewed by our esteemed Editorial Board for accuracy and currency.
Full spectrum of neurology in 1,200 comprehensive articles.
Listen to MedLink on the go with Audio versions of each article.
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
General Neurology
Dec. 28, 2024
Infectious Disorders
Dec. 28, 2024
Infectious Disorders
Dec. 27, 2024
Infectious Disorders
Dec. 12, 2024
Infectious Disorders
Dec. 10, 2024
Infectious Disorders
Dec. 10, 2024
Peripheral Neuropathies
Nov. 16, 2024
Infectious Disorders
Nov. 15, 2024