General Neurology
Use of focused ultrasound in neurologic disorders
Jan. 13, 2025
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Worddefinition
At vero eos et accusamus et iusto odio dignissimos ducimus qui blanditiis praesentium voluptatum deleniti atque corrupti quos dolores et quas.
When compared to other medical fields, interventional neuroradiology stands out not only as one of the youngest subspecializations but also as a field with compelling growth over the last 2 decades. The need to take care of patients with otherwise difficult-to-treat conditions; the rapid technical evolution of hardware and software; and the interdisciplinary collaboration of radiology, neurology, neurosurgery, and other fields have led to a versatile and exciting discipline. Although the need for randomized and controlled studies to objectify the benefits and risks of neurointerventional procedures continues, it is already clear that modern medicine cannot do without interventional neuroradiology.
|
• Minimally invasive, image-guided neurointerventions are elegant alternatives to surgery or long-term medication and provide treatment accessibility in cases that lack other therapeutic options. | |
|
• Minimally invasive, image-guided neurointerventions encompass a variety of indications and can be performed with fluoroscopy, ultrasound, CT, MRI, and angiography – or a combination of modalities. | |
|
• Rapid growth of the field, particularly in randomized trial data supporting neurointerventional treatments, has resulted in frequent updates to national and international guidelines and practice parameters. | |
|
• Neurointerventionalists and their patients rely on a dependable relationship with referring physicians as well as robust interdisciplinary collaboration between neuroradiology, neurology, and neurosurgery. |
Image-guided diagnostic procedures initially started in the late 19th century by utilizing x-ray to view swallowed contrast material for gastrointestinal imaging, which is a diagnostic test that remains important to this day (31). This was followed by x-ray images utilizing the intraspinous injection of air for contrast in a procedure called pneumoencephalography circa 1918 (35), and with injected intravascular contrast material, at first known as arterial encephalography, a decade later in 1927 (03). Expansion of the field from diagnosis to therapeutic intervention boomed after the development of CT imaging in the late 1960s and early 1970s, followed by minimally invasive CT-guided biopsy in the early 1980s (07; 36).
Angiography became safer and more accessible in the 1950s with the development of the Seldinger technique for catheterization of vessels, although cerebral angiography via direct carotid puncture continued until the 1970s as several technical problems first needed to be solved. From that point until the late 20th century, the neurointerventionalist was at the same time, an inventor and craftsman, having to individually fabricate rough and ready constructs of wires, catheters, and balloons for diagnosis and treatment. In contrast, today’s neurointerventionalists are part of a global community in close concert with material research and development. The tradition of teaching and research, as well as the remarkable evolution of high-quality equipment designed specifically for neurovascular pathologies, has brought the field remarkably forward since 1990 and made the treatment of complex disorders accessible and standardized. Nevertheless, the subject matter is intricate, and a thorough understanding of neuroanatomy, physiology, and pathology as well as experience with a variety of neurovascular conditions – including treatment and complications – is a prerequisite for successful case completion. Neurointerventionalists can be from a variety of medical backgrounds but most commonly originate from residency and fellowship training in neuroradiology, neurosurgery, or vascular neurology. The global collaboration of neurointerventionalists in the 21st century has led to tremendous growth in the evidence basis for the natural history and successful treatment of hitherto seldom occurring and difficult-to-treat conditions.
Interventional target. Interventional neuroradiology interventions target vessels (both arteries and veins), bones (with intervening joints and discs), tumors, and nerves.
Guidance. In contrast to open surgery, interventional neuroradiology procedures are characterized by indirect visualization of the interventional process by means of a radiological guidance modality. The intra-interventional imaging acquired thereby shows not only the position of the material used but also documents the course of the intervention, confirms treatment success, or indicates the occurrence of a complication. X-ray technologies such as fluoroscopy and CT are often used for guidance, but MRI and ultrasound can be used in certain situations as well.
Fluoroscopy. A continuous, real-time x-ray technique provides images of the body similar to a static x-ray; however, it includes the capability of visualizing organs or objects in motion, such as the diaphragm during breathing or a radiopaque catheter moving through a vessel. The patient is between an x-ray source and an x-ray detector. The image of the patient is made available on a display screen, and the frequency and length of the image acquisition are controlled by the operator with a foot pedal. All images are digitally stored (44).
Digital subtraction angiography. Digital subtraction angiography is a minimally invasive, fluoroscopic (live x-ray) procedure by which the vasculature is specifically evaluated (50). It consists of placement of a catheter into, for example, the femoral or radial artery utilizing the Seldinger technique. Under fluoroscopy, the catheter is then guided into the spinal, cervical, or intracranial vessels. A native (pre-contrast) bone mask is acquired followed by intravascular injection of contrast material through the catheter. The bone mask is actively subtracted from every post-injection image, resulting in a series of images that show only the vascular anatomy of interest (56). The same process can also be used for venous imaging via the jugular, femoral, or radial vein. The superiority of this technique is 3-fold: (1) in the superior spatial resolution (sub-millimeter) relative to other imaging techniques; (2) the temporal information obtained as contrast medium flows from arterial to capillary to venous phase; and (3) the capability for imaging one vascular territory at a time.
Roadmap. Roadmapping is a special digital subtraction angiography technique in which the interventionalist chooses an angiographic image in which the vascular territory of interest is delineated by an intravascular contrast agent. This image is then superimposed on real-time images of the moving catheter. The technique allows for catheter advancement without the need for continuous contrast injections.
Cross-sectional imaging.
Computed tomography (CT) / CT angiography (CTA). CT was developed by Sir Godfrey Hounsfield in the late 1960s. CT uses circumferential x-ray acquisition combined with a computer system that reconstructs the data into the transversal, coronal, sagittal, and 3D images with which we are familiar today. CT images can be augmented by intravenous, intra-arterial, oral, or intrathecal contrast medium. CT images are excellent for imaging of bone, acute blood, and the vessels. CT has acceptable soft tissue delineation. CTA is easily and readily acquired with intravenous contrast and rapidly provides images from the aortic arch to the intracranial circulation. Sensitivity and specificity for intracranial aneurysm detection is similar to MRI (33). CT is heavily used for image-guided intervention in the abdomen but also plays an important role in vertebroplasty (49) and a growing role in the interventional management of back pain (01).
Magnetic resonance imaging (MRI) / Magnetic resonance angiography (MRA). The first commercial MRI was available in 1980, followed by the first medical publications in the early 1980s. At that time, there were few sequence options and lengthy scan times. With the advent of parallel imaging techniques allowing for increased speed of acquisition, the landscape for MRI changed dramatically in the mid-1990s (15). MRI allows for excellent soft tissue contrast and, therefore, delineation of the effect of intracranial vascular pathologies on surrounding brain tissue, particularly acute ischemia. MRI is now used not only for typical luminal imaging of the vasculature with MRA but also for high-resolution vessel wall imaging. Sensitivity and specificity for intracranial aneurysm detection at 3 Tesla are similar to CTA (33). Although more expensive, MRI is far more advantageous for imaging of the spinal cord and preferable for detection of disc herniation and the effects of vertebral body degenerative changes on the spinal cord and nerves. MRI-guided procedures are not as frequent as those that are CT-guided, but they do exist for many neurologic indications (19).
A wide variety of materials is used in interventional neuroradiology procedures (45; 59). Some of the most common devices and their applications are discussed below.
Catheters. Catheters are thin tubes that are inserted into the body, usually into an artery or vein. Microcatheters have an internal caliber below 0.027 inches and allow for superselective catheterization in that the tip can be placed in smaller and more peripheral branches. Catheters are usually directed over microwires, or when specially designed, simply via blood flow.
Wires. Wires are used to help direct catheters or during the Seldinger technique. Wires vary in diameter and stiffness. Moreover, based on the material and construction of the wire, they can be hydrophilic or nonhydrophilic and have more or less torque. Typically, wires can be shaped by the interventionalist to facilitate access around curves.
Sheaths. Sheaths are short, stiff tubes, most commonly 11 cm long, placed by the Seldinger technique to establish reusable access to a vessel. The sheath is secured at the location of vessel puncture; catheters and wires can be safely inserted into and removed from the vessel through the sheath. Through the sheath, catheters can be maneuvered to desired vessels within the body, using imaging guidance.
Seldinger technique. Developed by Dr. Sven Seldinger (57), this is a technique in which a vessel is punctured with a hollow needle allowing passage of an atraumatic wire into the vessel. The needle is withdrawn, leaving the wire in place extending from inside the vessel to outside of the patient. A short sheath is passed over the wire into the vessel, and the wire is removed. The sheath can be secured and safely remain in place with appropriate saline flushing to prevent thrombosis.
Stents. Stents are tube-shaped mesh-like devices used to reopen a vessel and, in general, are designed with high radial outward force to keep the vessel open against pathologies that cause a stenosis or narrowing, such as atherosclerotic plaque. Drug-eluting stents are coated with medication to prevent in-stent thrombosis. Over time, most stents will be overgrown by endothelial cells from the vessel itself (endothelialization) (11). Stents with very little radial force can be used in conjunction with intracranial aneurysm coiling in the event of a wide aneurysm neck to prevent coil protrusion from the aneurysm into the parent vessel. Low radial force and retrievable stents are used to catch and remove embolic clots in mechanical thrombectomy for acute ischemic stroke.
Stent grafts. In addition to bare-metal or drug-coated stents, stent grafts are supplied with a polymer material covering the meshes of the stent, providing a sealing-off phenomenon not achievable by mesh stents. They are used for specific indications and typically in large vessels (39).
Flow-diverter stent. Flow-diverter stents have 30% more metal than routine bare-metal stents, however, less radial force than stents used to maintain vessel patency against a pathologic force such as atherosclerotic plaque. Flow-diverter stents are specifically and predominantly used to cover wide-neck aneurysms in the brain instead of placing coils within the aneurysm (13). These devices are increasingly incorporating specialized surface modification in order to reduce thrombogenicity and the need for post implantation dual antiplatelet medications (38).
Coils. Typically made from a platinum tungsten alloy, coils can be detachable or nondetachable (eg, pushable and so-called “liquid” coils) and induce vessel or aneurysm occlusion (67). Coils are thin metal wires manufactured with a pre-shaped configuration and are subsequently confined in a plastic linear housing for delivery into a catheter. After release or detachment, the preformed configuration causes the coil to assume a certain shape and size. Coils are usually chosen 20% to 30% larger in diameter than the target vessel. The expansion of the coils is hereby stopped by contact with the vessel wall, causing the coil to stay at the place of deployment. Whether coils are bare metal or contain fiber, the presence of enough coil density subsequently leads to blood clot formation and occlusion of the target vascular structure. The original concept of detachable coils as developed by Guglielmi and used first in man in 1990 (14) means that the coil can be retracted and repositioned until optimally placed and then detached by an electric current. Many other forms of detachment exist today, such as hydraulic and ball-and-socket methods.
Embolic agents. In contrast to coils, embolic agents travel in fluid and contrast substrate, called a slurry, with the blood flow distal to the catheter tip. Some embolic agents such as polyvinyl alcohol (PVA) particles and micro- or embospheres lead to permanent blood vessel occlusion and subsequent tissue necrosis. Others, such as gelatin sponge material, induce temporary blood vessel occlusion, which can be desired, for example, in an intervention aiming to reduce heavy epistaxis. Glues (eg, cyanoacrylate) and other non-glue embolic materials polymerize within the vessel lumen. The polymerization rate and the viscosity vary with the manufacturer and product; in the case of glue, these properties can be adjusted on the table by the practitioner (48; 32).
Sclerosants. There are a variety of chemical agents, including common medications like doxycycline, that can act as sclerosant to occlude and even destroy unwanted or pathologic blood vessels. The classic and most effective sclerosant is 98% absolute ethanol solution (ETOH), but it is also the most dangerous product for this purpose. It is only used by highly specialized and experienced practitioners. Ethanol is now available typically diluted and in more practical, viscous formulations such as being suspended in a cellulose gel. Ethanol and other sclerosing agents are commonly used for the ablation of vascular malformations or vascular tumors outside of the brain.
Nondetachable balloons. Compliant balloons are used for temporary vessel occlusion, and noncompliant balloons are used for angioplasty. Temporary placement of a compliant balloon can be used to facilitate more precise coil placement for treatment of wide-neck intracranial aneurysms, for example.
Endovascular procedures include the risk of complications at the access site or anywhere in the vasculature. These can range from rather minor puncture site hematomas requiring local compression only to more serious events such as arterial dissection, perforation, and pseudoaneurysm formation. Thromboembolic complications resulting in stroke as well as iatrogenic intracranial hemorrhage from vessel perforation are the most critical and immediate. Retroperitoneal hemorrhage from a complicated groin puncture can also be a life-threatening emergency. Most complications can be controlled with endovascular or surgical approaches.
Each device within a blood vessel is a foreign body that induces clot formation, partially explaining the risk of embolic stroke associated with endovascular procedures. Therefore, catheters are frequently flushed with heparinized saline in order to minimize thromboembolic complications. Besides clot formation, endovascular manipulation can also shear preexisting plaques from arterial walls or lead to formation and fragmentation of thrombus. Furthermore, irritation of the vessel wall frequently leads to vasospasm. Well-trained practitioners will recognize complications immediately and address them at the time of the intervention. Knowledge of drugs with special regard to those influencing coagulation is a part of overall management. Sufficient imaging and critical care capabilities as well as general, vascular, and neurosurgical coverage is essential for the management of complications.
Intracranial complications of endovascular procedures include hemorrhage in different locations, with subarachnoid hemorrhage being most frequent (usually arising from vessel perforation or aneurysm rupture). The occurrence of intracranial hemorrhage leads to the involvement of neurosurgery and usually placement of a ventricular drainage if one is not present already. Complications in context with material include acute or delayed in-stent thrombosis, usually requiring at least medical, and probably further, interventional treatment. Misplacement and displacement of material can occur because of incorrect sizing or underestimation of blood flow velocity. Embolic agents or sclerosants can be carried away by retrograde flow by unseen collateral vessels and be transported into an unwanted vessel. Learning to manage complications is an essential part of neurointerventional training.
Radiation. Since fluoroscopy and CT are the most frequently used imaging modalities for guidance in interventional neuroradiology procedures, the effects of ionizing radiation must be considered. They can be divided into deterministic and stochastic effects. Deterministic effects are characterized by a threshold of radiation dose below which they do not occur. Above the threshold level, the effect increases with dose. Skin and hair damage are typical examples (28; 04; 25). Risk factors for aggravated skin damage include recent radiation exposure, diabetes mellitus, preexisting rash, and connective tissue diseases, such as scleroderma and systemic lupus erythematosus. Stochastic effects of ionizing radiation are characterized by the absence of a threshold. There is also no linear relationship between dose and effect. However, the occurrence of stochastic effects is more likely with higher doses. Neoplasia is a typical example of stochastic effects (61). With regard to radiation applied to the central nervous system, radiation-induced neoplasia covers not only malignant tumors but also radiation-induced relatively benign tumors such as meningioma and cavernoma (62). With intention to foster patient safety, serious efforts have been achieved substantial reduction in radiation dose of diagnostic and interventional imaging in the past decade (18), both in terms of technological advances and awareness initiatives as well as dose recording and registries. During a single interventional neuroradiology procedure, the interventionalist team is exposed to a much lower radiation dose compared to the patient. However, the recurrence of exposure with every new case makes radiation protection a necessary part of interventional neuroradiology procedures. Lead gowns including thyroid gland protection and screens of lead glass belong to the standard equipment. Goggles with 0.5 mm of lead can aid in protecting the interventionalists’ lenses from opacities and cataracts (64). Extreme reactions to radiation can occur in patients with ataxia-telangiectasia (02).
Contrast agents. Endovascular interventional neuroradiology procedures usually require the repeated intravascular application of iodine-based contrast agents. Less frequently, patients receive gadolinium-based contrast agents during an intervention with MR guidance or during pre-interventional MR examinations for intervention planning. For both sorts of contrast agents, allergic reactions and hypersensitivity are known possible side effects. Contrast-induced nephropathy is an incompletely understood process (06); however, the most common cause is preexisting chronic kidney disease (41). Currently, most institutes limit the intravascular application of iodinated contrast agent to patients with renal function above a specified threshold. Some patients with renal function below the threshold might still be candidates for the procedure if the excretion of the contrast agent is accelerated by hemodialysis. Gadolinium-based contrast agents have been linked to nephrogenic systemic fibrosis, especially if linear instead of macrocyclic contrast agents are used and if the patient’s kidney function is reduced (66).
|
Condition |
Diagnostic |
Therapeutic |
Guidance |
Techniques |
Pre-interventional imaging |
|
Embolic stroke, especially large vessel occlusion |
X |
X |
DSA |
Thrombectomy: contact aspiration or stent retriever |
CT, MRI |
|
Atherosclerosis and stenosis |
X |
X |
DSA |
Balloon angioplasty |
CT, MRI |
|
Intracranial and spinal aneurysm |
X |
X |
DSA |
Coil or liquid embolization |
CT, MRI |
|
Intracranial vasospasm including reversible vasoconstriction syndrome |
X |
X |
DSA |
Intra-arterial medication injection |
CT, MRI |
|
Vascular malformations, such as arteriovenous malformation, dural arteriovenous fistula, carotid-cavernous fistula, vein of Galen malformation |
X |
X |
DSA |
Coiling or liquid embolization |
CT, MRI |
|
Subarachnoid hemorrhage of unknown origin |
X |
DSA |
Diagnostic angiography of the intracranial or spinal arteries |
CT > MRI | |
|
Sinus thrombosis |
X |
X |
DSA |
Venous thrombectomy |
CT, MRI |
|
Pituitary gland dys- or malfunction |
X |
DSA |
Inferior petrosal venous sinus sampling |
MRI | |
|
Nerve root pathology |
X |
X |
CT |
Perineural or epidural infiltration |
MRI |
|
Tumor: biopsy |
X |
CT > other |
Core needle biopsy |
MRI, CT | |
|
Tumor: treatment |
X |
CT, MR |
Ablation |
MRI, CT |
|
Condition |
Diagnostic |
Therapeutic |
Guidance |
Techniques |
Pre-interventional imaging |
|
Head and neck tumors, benign or malignant |
X |
DSA |
Particle or liquid embolization |
CT or MR | |
|
Extracranial vascular and lymphatic malformations |
X |
DSA, CT, ultrasound |
Intra-arterial, intravenous, and percutaneous sclerosants |
CT, MR, ultrasound | |
|
Retinoblastoma |
X |
DSA |
Intra-arterial chemotherapy |
MRI | |
|
Vertebral body fracture |
X |
CT, fluoroscopy |
Augmentation: vertebroplasty or kyphoplasty |
CT, MRI | |
|
Intervertebral disc pathology |
X |
CT, fluoroscopy |
Percutaneous discectomy and disc decompression, ablation, and sclerosing agents |
MRI |
Minimally invasive therapeutic options allow reach to anatomic areas that are not easily accessible through surgical approaches, allowing for local drug application and minimally invasive preoperative or even curative embolization. Occasionally, endovascular treatment may be the only option for some intracranial fistulas, in particular carotid-cavernous fistulas. For disease entities such as intracranial aneurysm embolization and thrombectomy for acute ischemic stroke, a comparable or even a superior outcome can be achieved if interventional neuroradiology therapeutic options are used instead of traditional surgical or systemic pharmacologic therapies.
The HERMES meta-analysis of positive randomized controlled stroke trials from 2016 confirmed a marked endovascular treatment benefit over medical therapy, including functional independence (20). Similar outcomes are seen with endovascular coiling versus microsurgical clipping of intracranial aneurysms; however, divergences arise when considering ruptured versus unruptured aneurysms and the size and location of the aneurysm (42; 60; 10).
For arteriovenous malformations, the situation is more complicated because interventional neuroradiology procedures are often intended to preoperatively reduce the size of the nidus and, thus, do not represent, generally, a standalone treatment (51). Several studies have reported on safety of embolization (37). In subgroups of patients who received intent-to-cure endovascular therapy, complete obliteration rates of up to 75% have been described (68).
A meta-analysis of 22 trials compared endovascular treatment with carotid endarterectomy and percutaneous transluminal balloon angioplasty and stenting for carotid artery stenosis (43). The risk of periprocedural stroke or death for patients younger than 70 years of age was similar for carotid artery stenting and carotid endarterectomy. However, for patients older than 70 years of age, balloon angioplasty and stenting were associated with a higher risk of stroke or death than endarterectomy.
Interventional neuroradiology requires a high level of skill and expertise and, as such, has received dedicated attention to training standards in the USA (29; 12) and Europe (53).
Specialized requirements also concern other members of the interventional team, such as the radiologic technologists and colleagues from anesthesia and nursing. Interdisciplinary collaboration with neurology, neurosurgery, and other subspecialties is essential.
Finally, interventional neuroradiology procedures often depend on special and cost-intensive hardware, including not only the materials used during the procedure but also a dedicated biplane angiography suite and access to other imaging modalities for planning, guidance, and follow-up. Many interventional neuroradiology procedures can only be performed safely if resources from anesthesia, intensive care, neurology, and neurosurgery are available when needed and, thus, require a full, large-scale medical center. Lower mortality rates have been demonstrated when patients with ruptured aneurysms are treated by experienced cerebrovascular neurosurgeons and neurointerventionalists in hospitals with larger volumes of cases and when care is provided in dedicated neurocritical care units (24).
The following quote published in a scientific statement from the American Heart Association reflects the commitment of the neurointerventional community to evidence based work (17):
|
“Endovascular neurointervention has continued to be a rapidly evolving field, with new devices and techniques expanding the range of diseases that can be treated and improving the safety of the therapies… there has been a corresponding acceleration of outcomes research that establishes the efficacy of these treatments and clarifies their role relative to pharmacological or open surgical therapies.” |
As mentioned, the speed of development of material and techniques often outruns the recruitment time necessary for randomized controlled trials, particularly with uncommon diseases and low patient numbers. Therefore, current material and techniques often lack high-level scientific evidence or even FDA approval, relying on expert opinion that guides societal recommendations.
Nevertheless, during the last years, an increasing number of large studies, often performed prospectively and with patient randomization, provide high-level evidence for the safety and efficacy of some interventional neuroradiology interventions, in particular:
|
• Endovascular treatment for emergent large vessel occlusion in acute ischemic stroke (20; 52) | |
|
• Coiling of unruptured and ruptured intracranial aneurysms (08; 60; 10) | |
|
• Stenting of carotid artery stenosis compared to carotid endarterectomy (43) |
Patient selection is of great importance in order to reduce the frequency of complications. Assessment of comorbidities, baseline functional status, natural history of the disease, and overall risk estimation should be included. Informed written consent is part of the standard of care. Similar to patient selection is the selection of the therapeutic approach and the choice of an alternative plans in case the first attempt does not lead to success.
Preprocedural planning. Anatomic considerations play an important role in preprocedural planning. Prior to endovascular treatment, knowledge about the exact location and orientation of the pathology as well as the adjacent vulnerable anatomy, including unseen potential collateral circulation, is of high importance. Awareness of these factors leads to accurate informed consent, the preparation of appropriate materials, proper anesthesia, and clear expectations of length of time of the procedure and the recovery. Preprocedural planning is especially important if it reveals the likelihood of a severe complication and subsequent reassessment of the therapeutic possibilities.
Interdisciplinary communication. Interventional neuroradiology relies greatly on interdisciplinary collaboration, and the importance of clear communication is not to be underestimated. Poor communication leads to confusion, uncertainty, or even frank misunderstanding. On the other hand, unambiguous communication aids in accelerating workflows, improving patient safety, and establishing an atmosphere of concentration. Standardized scales such as NIHSS, ASA Class, or ASPECT score render the exchange of information quick and unambiguous. Other standardized forms of communication, such as usage of the formal WHO surgical checklist during a team time-out, have been shown to reduce procedural complications and mortality (22).
Anesthesia for neurointervention. Diagnostic digital subtraction angiography can be performed with only local anesthesia at the access site or with light or moderate sedation. General or deep anesthesia is not always necessary for endovascular intervention. However, intubation is recommended for patients at risk of aspiration, patients in an unstable hemodynamic situation, and patients who cannot lie flat. Certain interventions require not only intubation but paralysis.
Lee and colleagues described “several anesthetic concerns that are particularly important for interventional neuroradiology procedures, including (1) maintaining immobility during procedures to facilitate imaging; (2) rapid recovery from anesthesia at the end of procedures to facilitate neurologic examination and monitoring or to provide for intermittent evaluation of neurologic function during procedures; (3) managing anticoagulation; (4) treating and managing sudden, unexpected, procedure-specific complications during procedures (eg, hemorrhage or vascular occlusion), which may involve manipulating systemic or regional blood pressures; and (5) guiding the medical management of critical care patients” (30). Many situations require the anesthesia team to be well informed about the pathophysiological processes associated with a patient’s disease and with the intervention taking place. For example, several randomized trials show noninferiority of general anesthesia versus moderate sedation for acute ischemic stroke when performed rapidly and without even transient hypotension, namely the SIESTA, AnStroke, and GOLIATH trials (54; 34; 55).
Pregnancy and motherhood. Nonemergent procedures, such as endovascular treatment of unruptured aneurysms, are usually scheduled after pregnancy and the postpartum period. Pregnant women are considered to be at increased risk of ischemic, thrombotic, and hemorrhagic stroke (26), and the puerperium is associated with increased risk of cerebral infarction and intracerebral hemorrhage (27) as well as cerebral venous thrombosis (58). However, pregnancy is considered a relative contraindication to intravenous thrombolysis in acute ischemic stroke. No systematic clinical trial has evaluated intravenous thrombolysis in pregnant women with ischemic stroke, but case reports about successful intravenous thrombolysis exist (21). Endovascular therapy remains an important option for these patients. Preeclampsia, eclampsia, and HELLP are important contributors to stroke during pregnancy and the postpartum period and need to be considered concerning stroke etiology and management (40). After delivery, postpartum angiopathy as part of the reversible cerebral vasoconstriction syndrome spectrum should be taken into account. Iodinated contrast agents and gadolinium-based contrast agents are known to be excreted into the maternal milk in small amounts. Some experts, therefore, recommend discontinuation of breastfeeding for 24 hours after intravascular contrast application and discharge of the maternal milk during this period.
Pediatric patients. Literature on endovascular neurointerventional procedures in children is scarce. However, equipment for endovascular procedures in children does exist, and case series reports on the endovascular therapy of ischemic stroke, intracranial aneurysms, and intracranial vascular fistulas have been published (16; 65; 05; 09). Recent articles indicate that endovascular therapy is safe in children (63). The transfer of diagnostic and therapeutic procedures from an adult patient population, in which these procedures are well established, to a pediatric population, in which they need to be labeled experimental, is common to many subspecialties of pediatrics. Due to the absence of controlled, prospective studies of neurointerventions in pediatric patients, these procedures fall under the same category. Many tissues of pediatric patients are at higher risk of radiation side effects compared to the same tissues in adult patients; therefore, special attention should be given to radiation safety in pediatric patients.
Vein of Galen malformations represent severe cerebrovascular malformations in the newborn. Their treatment heavily relies on endovascular therapy. Reduction of blood flow and consecutive improvement of cardiac output failure are often the most important goals of treatment (23). Postoperative hemorrhage is a known and possibly critical complication of tonsillectomy. Endovascular therapy can aid in controlling the situation (46).
Clinical history. A 91-year-old female patient presented to the emergency department with acute weakness on the left side and a subsequent fall. Structured neurologic examination revealed a brachiofacial, sensorimotor hemisyndrome on the left side and a hemineglect on the left side. Total National Institute of Health stroke score (NIHSS) was 14.
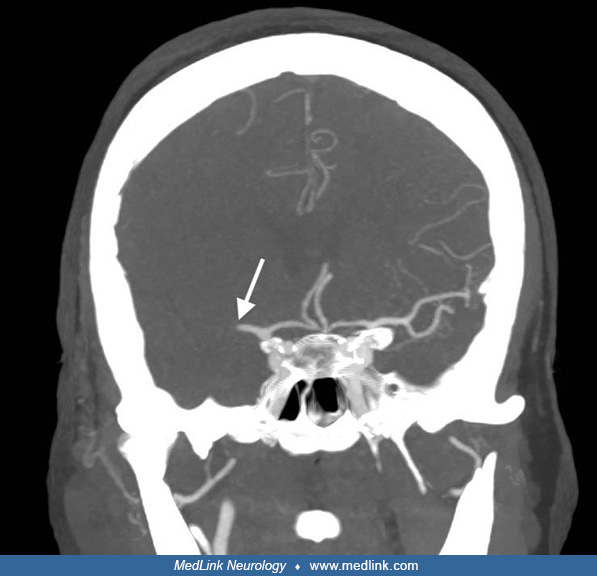
Imaging. Noncontrast CT of the head ruled out intracranial hemorrhage. A hyperdense artery sign of the M1 segment of the right middle cerebral artery was identified.
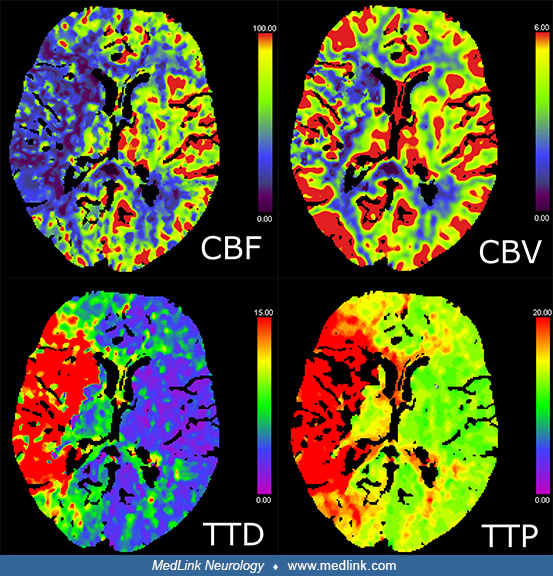
No loss of gray-white differentiation in terms of early ischemia was present. CT perfusion of the brain revealed a large territorial perfusion delay (prolonged time to peak), including the whole middle cerebral artery territory in the right hemisphere. Cerebral blood flow was correspondingly reduced in this territory. However, cerebral blood volume was mostly normal in the right hemisphere, indicating appropriate autoregulatory response and hyperemia. Only a small area of reduced cerebral blood volume was present in basal ganglia and the internal capsule.
CT angiography of the brain showed abrupt discontinuation of contrast filling in the M1 segment of the right middle cerebral artery, which was interpreted as acute occlusion. There were no sequelae of the traumatic fall.
In summary, imaging showed an acute M1 occlusion with a very small infarct core and a large ischemic penumbra.
Management. Intravenous thrombolysis was started immediately after noncontrast imaging. The neurointerventionalist in charge was consulted, and a decision in favor of emergent endovascular therapy was reached. The patient was transferred to the angiography suite while under permanent cardiopulmonary monitoring.
Intervention. The intervention was carried out under moderate sedation and monitored anesthesia care. The right common femoral artery was punctured using Seldinger technique, and the guide catheter was advanced into the right common carotid artery. A contrast injection confirmed the M1 segment occlusion.
After an initial unsuccessful attempt of revascularization via a retrievable stent, the clot was removed via direct contact aspiration. Subsequent injections confirmed the successful thrombectomy (thrombolysis in cerebral infarction score 3).
The arterial access site was closed. A noncontrast CT scan using the flat-panel detector found no intracranial hemorrhage.
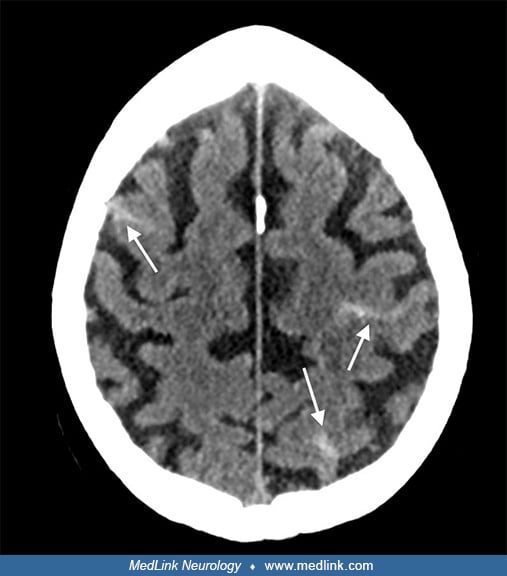
Further course. Post-interventional neurologic examination revealed NIHSS of two (minor dysarthria and ataxia) and further normalized within the next 2 days. The patient was transferred to the stroke unit for post-interventional surveillance. A cerebral MRI on the next day showed small infarcts in the right basal ganglia and a minimal infarct in the right parietal lobe.
Further diagnostic procedures followed with the intention to characterize the cause of stroke.
Take-home messages.
• The utilization of standardized scales such as NIHSS can aid in precise communication for rapid and algorithmic decision-making. | |
• Direct contact thrombus aspiration and the use of retrievable stents represent two variations of thrombectomy. |
Clinical history. A 67-year-old female patient presented to the emergency department with two episodes of sudden intense headache, dizziness, and nausea within the last 2 weeks. She was taking rivaroxaban (Xarelto) for deep venous thrombosis and pulmonary emboli. GCS was 15. NIHSS was 0.
Imaging. Initial noncontrast CT of the head was normal. Due to the suspicion of subarachnoid hemorrhage, lumbar puncture was performed, and laboratory analysis of CSF yielded xanthochromia and increased amounts of erythrocytes and protein. Repetition of the CT on the next day showed new subarachnoid hemorrhage within three supratentorial sulci.
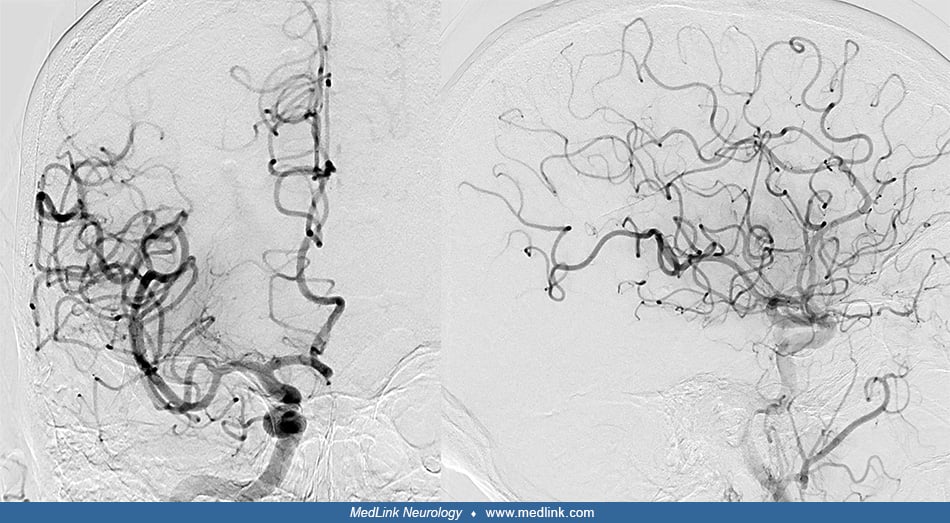
By performing CT angiography, irregularities of the caliber of the secondary divisions of the right middle cerebral artery and both posterior cerebral arteries were discovered. No intracranial aneurysm was visualized. An epileptic seizure on the same day subsequently led to a third CT, which revealed new areas of supratentorial sulcal subarachnoid blood. An invasive, primary diagnostic angiography was requested in order to search the etiology of subarachnoid hemorrhage and to verify the vessel irregularities.
Intervention. The intervention was carried out under moderate sedation and monitored anesthesia care. The right common femoral artery was punctured using the Seldinger technique, and the guide catheter was advanced into the right common carotid artery. Contrast injections into both internal carotid arteries revealed predominantly peripheral caliber reductions of several intracranial arteries, compatible with vasoconstriction--severe at M3- and P3-segments of the right middle cerebral artery and posterior cerebral artery, respectively, and moderate at the distal left posterior cerebral artery.
No aneurysm or vascular malformation was found. Together with the clinical history of sudden, severe headache, multiple medications for a psychiatric disorder as well as a long-term history of multiple surgeries and cycles of chemotherapy for a mucinous adenocarcinoma of the appendix, the diagnosis of reversible cerebral vasoconstriction syndrome was established. Slow intra-arterial application of verapamil, a calcium channel blocker, into both internal carotid arteries followed. Subsequent contrast injections documented a substantial decrease in the areas of vasoconstriction (47).
Further course. The patient was transferred to the intensive care unit for surveillance and supportive care. The medication with verapamil was continued by oral administration of 60 mg Nimotop, six times per day. Post-interventional MRI of the head revealed the known subarachnoid hemorrhage and confirmed the absence of infarcts.
Daily transcranial doppler was performed without evidence of proximal vasospasm, and the dose of verapamil was reduced. The patient’s headache improved, and she remained without further seizure or focal neurologic deficits. She was discharged to a rehabilitation hospital in a stable general state of health.
Take-home messages.
• In the presence of unexplained nontraumatic sudden and severe headache with a pattern of “cortical” or “convexity” subarachnoid hemorrhage, the differential diagnosis includes many causes that may require catheter angiography if the initial cross-sectional imaging is reported as negative. Catheter angiography is the reference standard for vascular imaging in terms of both spatial and temporal resolution. It is superior to CTA and MRA for the detection of small and/or distal intracranial aneurysms as well as for the detection of small arteriovenous malformations and arteriovenous fistulas; in any case, catheter angiography is required for the grading of any vascular malformation. | |
• Besides the application of coils, stents, or embolic material, microcatheters can also be used for the intra-arterial application of drugs within a target vessel. Apart from calcium channel blockers, as in this case, thrombolytics and chemotherapy are also delivered intra-arterially. |
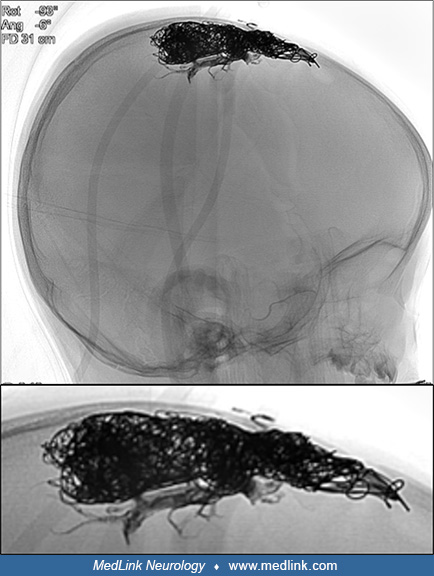
Clinical history. A 2-year-old child presented to the emergency room with sudden decrease in consciousness and had a history of head circumference at the upper range of normal. The child was otherwise normal and had reached expected milestones. There was no history of perinatal or birth problems.
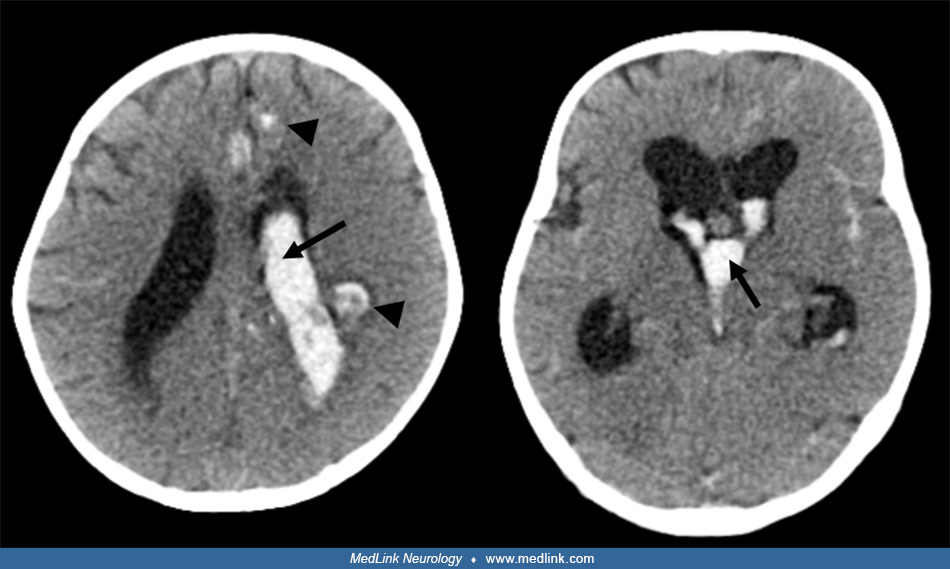
Imaging. Initial noncontrast CT of the head showed intraventricular hemorrhage within enlarged ventricles.
A subsequent MRI showed abnormal dilated supratentorial vessels.
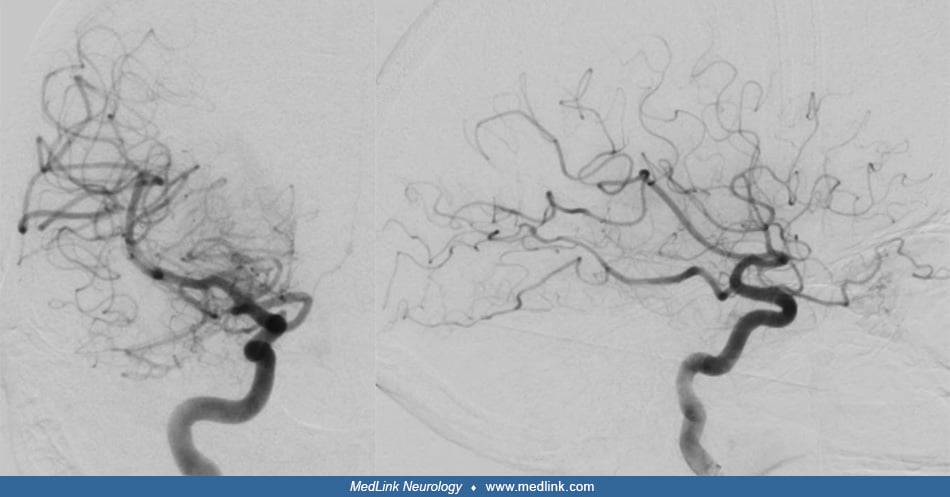
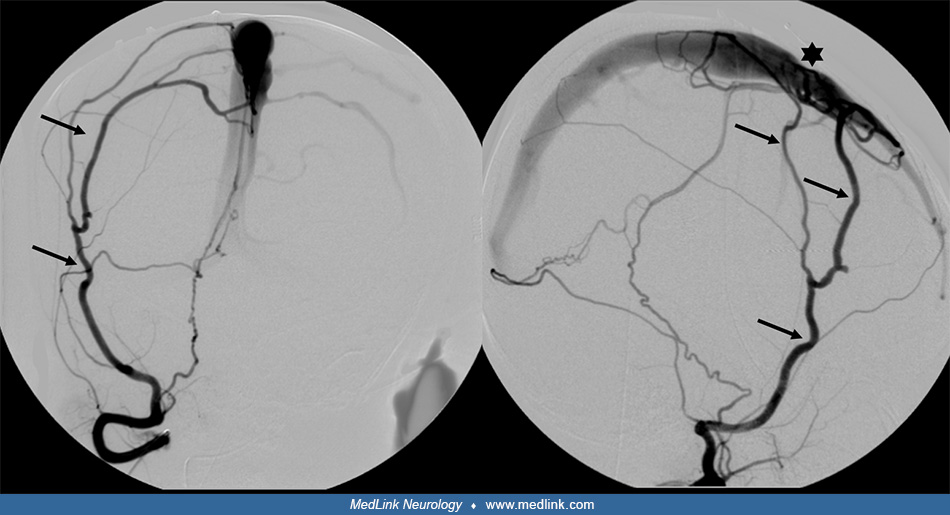
Angiography delineated a high-flow arteriovenous shunt predominantly from the external carotid artery via the middle meningeal arteries directly through a fistula point into the middle third of the superior sagittal sinus.
Because of the arterial flow in the dural venous sinuses – the superior sagittal sinus and both transverse and sigmoid sinuses – the venous drainage of the brain had accommodated by redistributing anteriorly to the cavernous sinus and other collateral pathways.
Intervention. Using a transvenous approach, the superior sagittal sinus was occluded by coils at the site of the fistula. Subsequently, via transarterial access, the distal middle meningeal arteries were accessed, and liquid embolic material was injected around the coils and retrograde into other feeding dural arterial branches until the fistula was finally occluded.
Further course. The child tolerated the procedure well, and there were no immediate complications. The patient was discharged to home after several days of anticoagulation and observation. At 1-month follow-up, MRI showed that there was thrombosis of the dural venous sinuses; however, this had no clinical significance, and there were no venous infarcts. There was a complete recovery.
Clinical history. A 59-year-old female patient presented to the emergency department with complaints of new onset of back pain and slight loss of control of the right leg.
Imaging. MRI of the lumbar spine revealed a foraminal disc protrusion with compression of the L4 spinal nerve on the right side.
There was also partial lumbarization of S1. An ambulant periradicular infiltration was scheduled.
Intervention. The patient was positioned on the left side on the CT table, and a noncontrast CT scan was obtained. The intended needle path from the skin to the right L4 spinal nerve was planned utilizing guidance software.
The software directs a focal beam of red light indicating the puncture site and needle angle. After disinfection and sterile covering, local anesthesia was applied at the puncture site. Finally, a 22G coaxial needle was placed next to the nerve root using repetitive CT scans to aid in needle tip positioning.
After confirmation of correct needle positioning, a mixture of bupivacain (local anesthetic), dexamethasone (glucocorticoid), and Iopamiro (iodine-based CT contrast agent) was injected around the spinal nerve. A final CT scan proved the iodine-based contrast agent to be located around the spinal nerve within the extradural space.
Because the three substances had been mixed before injection, an inadvertent intradural or intravascular injection of the local anesthetic or the glucocorticoid could be excluded.
Further course. After 1 hour of bed rest, the patient was examined. She reported a clear improvement concerning the back pain. The strength of both legs was found to be symmetric and normal. Inspection of the puncture site revealed no evidence of hemorrhage. The patient was discharged.
Two weeks after the periradicular therapy, the patient presented again to the emergency department. She reported a good effect of the intervention for 1 week, but symptom recurrence in the second week. The initial success of perineural infiltration in conjunction with the known foraminal disc protrusion allowed her symptoms to be interpreted as radiculopathy. This formed the basis for the following discussion of therapeutic options.
Take-home messages.
• In confirming the source of pain, perineural infiltrations and intra-articular injections often have a diagnostic function. | |
• Anatomic information such as the presence of a lumbosacral junction variation should be known prior to intervention in order to minimize the risk of injection at the wrong level. | |
• CT-guided periradicular drug infiltrations represent a higher level of anatomic guidance as compared to fluoroscopically guided infiltrations. |
Clinical history. A 73-year-old female patient presented with progressive incomplete paraparesis. She complained about weakness and numbness of both legs for a month and upper back pain. At presentation, she was not able to walk.
Imaging. MRI of the whole spine revealed several vertebral body masses with fatty marrow replacement. The mass in T7 replaced the entire vertebral body with intraspinal epidural extension and displacement and flattening of the spinal cord, as well as lateral soft tissue extension.
Focal myelopathy signal was noted at this location. Differential diagnoses included metastases of a solid primary tumor, myeloma, and lymphoma. Interdisciplinary case discussion led to the decision of surgical decompression with simultaneous biopsy for histopathology. Due to the vascularity of the mass, a preoperative angiography was requested in order to outline major arterial feeders with optional embolization where appropriate.
Intervention. The intervention was carried out under moderate sedation and monitored anesthesia care. The right common femoral artery was punctured using the Seldinger technique, and the guide catheter was advanced into the descending aorta. Selective catheterization of the right and left segmental arteries from T5 to T8 bilaterally with consecutive injection of contrast agent showed an extensive tumor blush from the right segmental artery of T7.
A microcatheter was positioned in the segmental artery of T7, and particles of 250 microns in a contrast-saline slurry were injected. Post-embolization angiography revealed a considerable decrease in tumor blush.
The patient was prepared for surgical debulking and dorsal fusion the same day.
Further course. During surgery, the soft tumor tissue’s propensity to bleed was easily managed, and the intraspinal components of the mass could be resected. Histology revealed that the mass was a manifestation of plasma-cell myeloma. Seven days later, complete improvement of weakness and numbness of both legs was reached, and the patient was able to walk again. Further therapy of the myeloma included systemic chemotherapy and radiation of the affected segments of the spine.
Take-home message. In vascular tumors, preoperative embolization can simplify the limit intraoperative blood loss, particularly for tumors in delicate locations or in a surgical approach with limited possibility for hemostasis.
All contributors' financial relationships have been reviewed and mitigated to ensure that this and every other article is free from commercial bias.

Kristine Ann Blackham MD
Dr. Blackham of University Hospital of Basel in Basel, Switzerland, has no relevant financial relationships to disclose.
See Profile
Peter J Koehler MD PhD
Dr. Koehler of Maastricht University has no relevant financial relationships to disclose.
See ProfileNearly 3,000 illustrations, including video clips of neurologic disorders.
Every article is reviewed by our esteemed Editorial Board for accuracy and currency.
Full spectrum of neurology in 1,200 comprehensive articles.
Listen to MedLink on the go with Audio versions of each article.
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
General Neurology
Jan. 13, 2025
General Neurology
Jan. 13, 2025
Neuro-Ophthalmology & Neuro-Otology
Jan. 08, 2025
Neuro-Ophthalmology & Neuro-Otology
Jan. 07, 2025
General Neurology
Dec. 30, 2024
General Neurology
Dec. 13, 2024
General Neurology
Dec. 13, 2024
Neuromuscular Disorders
Dec. 09, 2024