Epilepsy & Seizures
Tonic status epilepticus
Jan. 20, 2025
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Worddefinition
At vero eos et accusamus et iusto odio dignissimos ducimus qui blanditiis praesentium voluptatum deleniti atque corrupti quos dolores et quas.
Myoclonic-atonic seizures are a particular type of brief and abrupt generalized epileptic seizure in which the myoclonic jerk is immediately followed by atonia. In polygraphic video EEG, the myoclonic jerk is correlated with a generalized 1 to 3 Hz spike/polyspike discharge and the atonia with a generalized slow wave. This type of seizure generates severe drop attacks that are often traumatic because the atonia prevents any protective movements. Myoclonic-atonic seizures should be differentiated from other types of seizures causing drop attacks, such as tonic, atonic, and myoclonic seizures and epileptic spasms. Myoclonic-atonic seizures occur nearly exclusively in children, and they are the defining seizure type of a genetic “epilepsy with myoclonic-atonic seizures.” Patients with myoclonic-atonic seizures also suffer from concurrent tonic, atonic, absence, and other types of epileptic seizures according to the primary epileptic syndrome. Management is usually difficult, and prognosis is that of the underlying disorder. In this article, the author details the clinical manifestations, pathophysiology, EEG, and optimal management of patients with myoclonic-atonic seizures.
|
• Myoclonic-atonic seizures are brief (approximately one second) and abrupt and manifest with a myoclonic symptom followed by an atonic symptom in continuity. | |
|
• The ictal EEG, in polygraphic video-EEG-EMG, manifests with a high-amplitude, 1 to 3 Hz generalized spikes and poly-spikes discharges associated with the myoclonic jerk followed by a slow wave associated with the loss of muscle tone. | |
|
• They usually occur in children who also have other types of seizure, such as atonic, myoclonic, tonic, or generalized tonic-clonic convulsions, and they usually occur after the onset of other seizures. They are the defining seizure type of epilepsy with myoclonic-atonic seizures, but they also occur in Lennox-Gastaut syndrome and other severe epilepsies of childhood. | |
|
• Prognosis is that of the epileptic syndrome with which these seizures occur. | |
|
• Treatment is that of the syndrome; the most appropriate combination is with valproate and lamotrigine. Ethosuximide (absences) and clonazepam (myoclonic jerks) may be useful. Ketogenic diet or modified Atkins diet is highly recommended in drug-resistant cases and those rare cases found to have glucose transporter 1 (GLUT1) deficiency. | |
|
• Contraindicated drugs include carbamazepine, oxcarbazepine, vigabatrin, phenytoin, and phenobarbital. |
Myoclonic-atonic seizures, brief and abrupt, are characterized by a myoclonic followed by an atonic symptom in continuity. Symmetrical myoclonic jerks of the arms or facial twitching precedes the more or less pronounced loss of tone (atonia).
The first clinical description of atonic seizures was given by Ramsay Hunt in 1922 as “a type of epilepsy characterized by a sudden loss of postural tone,” which he called a “static fit” (28). In 1945, Lennox called it “akinetic” (41), but he renamed it “astatic” in 1951 and included it into the “petit mal triad” together with atypical absences and myoclonic jerks (42). However, it was not before Gastaut and Regis’ polygraphic description that a specific type of seizure with a combination of myoclonus preceding atonia was reported (25): “We can in fact show that inhibition of muscle tonus can immediately follow the myoclonias of petit mal and may be so pronounced as to cause a fall. In this case it is in fact a postmyoclonic amyotonia that causes an akinetic fall” (25). Kruse coined the term “myoclonic-astatic” seizure for this type of combined epileptic seizure (39). In the 2010 ILAE classification, the term used was “myoclonic-atonic” seizure (02).
The latest ILAE position paper of the operational classification of seizure types considers myoclonic-atonic seizures as a new type of seizures and classifies them as motor generalized seizures together with tonic-clonic, clonic, tonic, myoclonic, myoclonic-tonic-clonic, atonic, and epileptic spasms (23; 22). The currently accepted ILAE instruction manual for the operational classification of seizure types describes myoclonic-atonic seizures as “a generalized seizure type with a myoclonic jerk leading to an atonic motor component. This type was previously called myoclonic-astatic” (22).
According to the ILAE Glossary the relevant definitions are (05):
Atonic seizure. A sudden loss or diminution of muscle tone without an apparent preceding myoclonic or tonic event, lasting approximately one or two seconds, and involving the head, trunk, jaw, or limb musculature (05). Atonic seizures are not synonymous with astatic seizures.
Astatic seizure (drop attack). A loss of erect posture that results from an atonic, myoclonic, or tonic mechanism (05).
Thus, an atonic seizure could also be called an astatic seizure, but not all astatic seizures are atonic as they may also be myoclonic or tonic-astatic. Furthermore, atonic seizures are not akinetic seizures. In akinetic seizures, there is an inability to perform voluntary movements that is not caused by loss of consciousness (as, for example, in absence seizures) or by loss of muscle tone (as in atonic seizures).
Atonic seizures may occur in continuation with a preceding myoclonic seizure; these are so-called myoclonic-atonic seizures.
Myoclonic-atonic seizures often occur in epilepsies with onset before the age of five years and predominate in epilepsy with myoclonic-atonic seizures. Doose and colleagues introduced the concept of a specific clinical entity with this type of seizure being the core of a disorder, which he called “centrencephalic myoclonic-astatic petit mal” (15; 18). This is accepted as an epileptic syndrome by the ILAE, initially under the name “myoclonic-astatic epilepsy” (12) and more recently “epilepsy with myoclonic-atonic seizures” (02; 11). It is also referred as “Doose syndrome” (34), particularly for the pure form of genetic nonstructural epilepsy with myoclonic-atonic seizures (57).
The ILAE Task Force considered “epilepsy with myoclonic-astatic seizures” an idiopathic generalized epilepsy (21), which is similar to Doose’s opinion that “epilepsy with myoclonic-astatic seizures belongs to the epilepsies with primarily generalized seizures and, thus, stands in one line with absence epilepsies, juvenile myoclonic epilepsy, as well as the infantile and juvenile idiopathic epilepsy with generalized tonic clonic seizures” (16). This contrasts markedly with (a) the 1989 ILAE classification in which epilepsy with myoclonic-astatic seizures was listed as a “cryptogenic/symptomatic” generalized epilepsy in the same group of disorders as Lennox-Gastaut syndrome (12), and with (b) the ILAE epilepsy diagnosis manual where epilepsy with myoclonic-atonic seizures is considered as epileptic encephalopathy (11).
In the 2014 ILAE “Epilepsy Diagnosis” manual (11), myoclonic-atonic seizures are categorized as one of the four types of generalized myoclonic seizure (myoclonic, negative myoclonus, myoclonic-atonic, and myoclonic tonic) and are described as follows:
|
A myoclonic-atonic seizure is a myoclonic seizure followed by an atonic seizure. Sometimes a series of myoclonic jerks occurs prior to the atonia. The head and limbs are affected, typically resulting in rapid fall. The myoclonic jerk may be subtle. NOTE: Myoclonic-atonic seizures are one type of seizure that can result in a "drop attack" (also known as astatic seizure), other causes of drop attacks include myoclonic (especially in younger children), tonic and atonic seizures. Ictal EEG: The myoclonic component is associated with a generalized spike or polyspike. The atonic component is associated with the aftergoing high voltage slow wave. CAUTION: Focal discharges are not seen. If they occur, then consider structural brain abnormality. Differential diagnosis: Atonic seizure, Tonic seizure, Typical absence: with myoclonic or atonic components. Related syndromes: Epilepsy with myoclonic-atonic seizures (11). |
This excellent epilepsy diagnosis manual also provides three video examples of myoclonic-atonic seizures.
In 2021, the ILAE Task Force on Nosology and Definitions proposal on epilepsy syndromes classification included “epilepsy with myoclonic-atonic seizures (EMAtS)” in the childhood epilepsy syndromes with generalized seizures, under the category of genetic generalized epilepsies. The term “epilepsy with myoclonic-atonic seizures (EMAtS)” is proposed to replace the previous term “Doose syndrome.” The proposal is currently at the public comment stage before it is finalized (60).
Myoclonic-atonic seizures usually occur in children (2 to 6 years of age, range 6 months to 8 years). A quarter to 38% of children have a history of febrile seizures, and the development prior to seizure onset is typically normal in two thirds of these children (61; 32). Developmental deficits may, however, be seen in a subset of these patients prior to onset. Boys are more commonly affected (62). The onset can be abrupt (“stormy phase”) with many seizures or may be gradual (32; 60).
Myoclonic-atonic seizures are the defining seizure type of epilepsy with myoclonic-atonic seizures (16; 50). In a genotype-phenotype study of 101 patients with epilepsy with myoclonic-atonic seizures, myoclonic-atonic or atonic seizures were seen in 100%, generalized tonic-clonic in 72%, myoclonic in 69%, absence seizures in 60%, and tonic seizures in 19% (61). The appearance of tonic seizures usually heralds a poorer long-term prognosis (60). A history of epileptic spasms or hypsarrhythmia prior to diagnosis is considered n exclusionary criterion (60). In 21% of patients, evidence of developmental and epileptic encephalopathy was present prior to epilepsy onset. Neurodevelopmental deficits eventually were reported in 62% of patients; 24% had autism spectrum disorder, and 37% had attention-deficit/hyperactivity disorder (61).
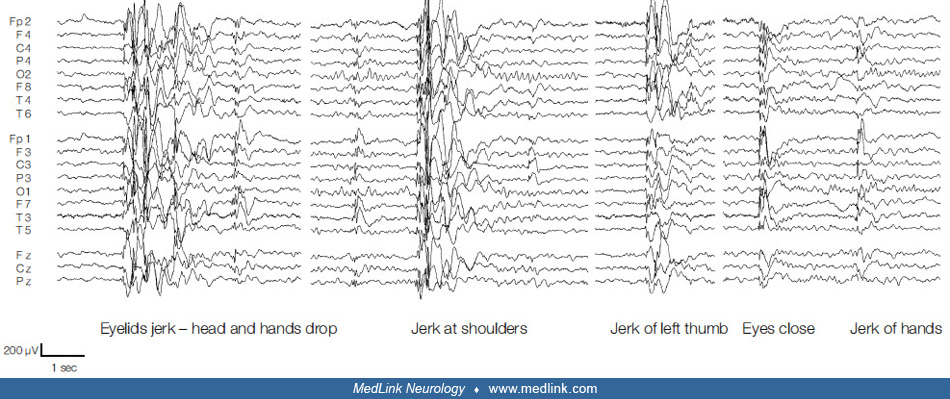
A typical myoclonic-atonic seizure consists of a myoclonic jerk of the upper limbs or trunk immediately followed by an atonic fall straight down, with the patient landing on the buttocks (53; 54; 55; 64). There is no propulsive or retropulsive falling; it is like the collapse of a marionette when all its strings are simultaneously cut. The whole episode lasts approximately one second or less, and the patient erects quickly from the floor with no impairment of consciousness.
In falls from the standing position, the patient suddenly flexes at the waist and knees, followed by further knee flexion, and then drops straight down and lands on the buttocks. When sitting, the patient falls forward or backward depending on the position of the center of gravity.
The seizure is abrupt, with no warning, which is also the reason resultant trauma is frequently seen.
There is a great variability of the intensity and extent of the myoclonic jerks preceding atonia; the jerks sometimes are difficult to detect without video-EEG polygraphic recordings. The jerks may be limited to the facial or neck muscles. Similarly, the atonic manifestations may be slumping of the head (head drops, head nodding), and upper trunk without falls. The same patient may have myoclonic-atonic seizures with all variations of intensity from mild to severe.
The prognosis depends on the epileptic syndrome. It varies from good to moderate or bad in epilepsy with myoclonic-atonic seizures where early onset of seizures and EEG focal spike discharges are indicators of poor prognosis (29). Prognosis is usually worst in Lennox-Gastaut and Dravet syndrome. In epilepsy with myoclonic-atonic seizures, although seizures may be drug-resistant at first, remission may be seen in two thirds within 3 years from onset (60). Developmental deficits or regression may appear, but improvement can be seen with effective treatment of epilepsy. Worse prognosis has been reported when tonic seizures or nonconvulsive status epilepticus are present, or if abundant spike-wave or slow spike wave complexes or generalized paroxysmal fast activity is seen (60).
A boy with normal neurocognitive development experienced at age two years a febrile generalized tonic-clonic seizure. Two months later he started having frequent febrile and nonfebrile generalized tonic-clonic seizures that were often prolonged. There was a family history of idiopathic generalized epilepsies. Interictal EEG had normal background, but there were frequent brief generalized discharges of 2.5 to 3 Hz spike/polyspike and slow wave, mainly in sleep. Treatment with valproate had an initial beneficial effect on the generalized tonic-clonic seizures, but three months later, the boy started having myoclonic jerks, absences, and falls--some of which were traumatic. Despite adding lamotrigine and later clonazepam, multiple seizures continued on a daily basis, and the boy also had lengthy episodes of myoclonic-atonic status epilepticus. Polygraphic video-EEGs documented myoclonic, absence, and myoclonic-atonic seizures.
Epileptic seizures improved in frequency and severity after four years of age and finally remitted at five years of age. The boy has mild cognitive impairment. He attends mainstream school with poor performance.
Localization is discussed in the pathophysiology section.
The main etiology of myoclonic-atonic seizures is detailed in the MedLink Neurology article Epilepsy with myoclonic-atonic seizures.
The localization and pathophysiology of myoclonic-atonic seizures is uncertain. The extent of involvement and the relevant contribution of the brainstem, thalamus, and cortex remain a matter of debate. However, it is definite that the myoclonic component associated with the generalized spike-polyspike is an excitatory motor phenomenon whereas the atonic component associated with the slow wave is an inhibitory phenomenon.
Pathophysiological considerations of the myoclonic components. The ILAE Commission on Pediatric Epilepsy considers that thalamocortical myoclonus occurs in:
|
• Idiopathic generalized epilepsies, such as benign myoclonic epilepsy of infancy and juvenile myoclonic epilepsy. | |
|
• Myoclonic absence seizures where a combination of positive and negative myoclonus exists; the muscle jerk is associated with the positive component of a spike that precedes its negative transient, whereas negative myoclonus follows the spike by 100 ms, and its onset is before the onset of the slow wave. | |
|
• Dravet syndrome | |
|
• Epilepsy with myoclonic-atonic seizures (currently proposed term: “myoclonic-atonic epilepsy”) | |
|
| |
A study of the neurophysiologic characteristics of epileptic myoclonus in patients with Lennox-Gastaut syndrome and epilepsy with myoclonic-atonic seizures showed considerable differences between the two syndromes. Epileptic myoclonus in Lennox-Gastaut syndrome appeared to originate from a stable generator in the frontal cortex and then spread to contralateral and ipsilateral cortical areas whereas myoclonus in epilepsy with myoclonic-atonic seizures appeared to be a primarily generalized epileptic phenomenon. In the latter, muscles from both sides were activated synchronously, and the EEG correlate was a generalized spike-wave in which the negative peak of the spike preceded the generalized jerks by 30 +/-2 ms (mean +/- SD). Topographic voltage mapping of the pre-myoclonic spike peak showed a diffuse distribution of the electrical field, predominating over the anterior regions but not lateralized (06).
Moeller and associates aimed to identify neuronal networks underlying generalized spike and wave discharges in myoclonic atonic epilepsy (46). Simultaneous EEG-fMRI recordings were performed in 13 children with this epileptic syndrome. Individual generalized spike-wave discharges-associated blood oxygenation level-dependent (BOLD) signal changes were analyzed in every patient. A group analysis was performed to determine common syndrome-specific hemodynamic changes across all patients. Generalized spike-wave discharges were recorded in 11 patients, all showing generalized spike-wave-associated BOLD signal changes. Activation was detected in the thalamus (all patients), premotor cortex (six patients), and putamen (six patients). Deactivation was found in the default mode areas (seven patients). The group analysis confirmed activations in the thalamus, premotor cortex, putamen, and cerebellum and deactivations in the default mode network. The authors concluded that in addition to the thalamocortical network, which is commonly found in idiopathic generalized epilepsies, generalized spike-wave discharges in patients with epilepsy with myoclonic-atonic seizures are characterized by BOLD signal changes in brain structures associated with motor function. The results are in line with animal studies demonstrating that somatosensory cortex, putamen, and cerebellum are involved in the generation of myoclonic seizures. The involvement of these structures might predispose to the typical seizure semiology of myoclonic jerks observed in epilepsy with myoclonic-atonic seizures (46).
Pathophysiological considerations of the atonic components. The pathophysiology of focal and generalized atonic seizures and the atonic components of seizures are largely unknown; cortical and subcortical mechanisms have been implicated to explain the loss of muscle tone (63).
Blume postulated that discharges in the premotor cortex and other areas, as reflected by spikes and troughs of bisynchronous spike-and-wave discharges, descend to excite the pontine and medullary reticular formation, which inhibits spinal motor neurons, thereby producing sudden hypotonia of axial muscles (04).
Velasco and colleagues studied centromedian thalamic nuclei epileptiform EEG activities recorded in children with intractable generalized seizures of Lennox-Gastaut syndrome through implanted recording-stimulating electrodes used for seizure control (66). Ictal thalamic epileptiform activities were consistently correlated to widespread surface cortical EEG activities and symptoms in all patients and all types of generalized seizures: fast spike discharges at thalamus correlated at onset of tonic and tonic-clonic generalized seizures; slow (1 to 2 Hz) spike-wave complex discharges at thalamus correlated for atypical absence seizures; slow polyspike-wave complex discharges correlated for myoclonic seizures; and spike bursts and suppression patterns correlated for combined tonic-atonic-myoclonic seizures. Ictal EEG activities occurred simultaneously at right and left thalamic electrodes and surface at onset of all seizure types, with the exception of myoclonic seizures in which thalamic complete discharges and individual spike-wave complexes significantly lead those of the surface. In combined tonic-atonic-myoclonic seizures, thalamic epileptiform activities consisted of high amplitude spike bursts and suppression-pattern discharges, simultaneously occurring with variable amplitudes in right and left thalamic electrodes and surface regions at onset of the spontaneous or evoked combined tonic-atonic-myoclonic seizures. These discharges lasted from 10 to 18 seconds (average 12.2 seconds), showing poor individual correlations between thalamic and surface discharges while patients showed repetitive generalized muscular spasms and sudden loss of muscular tone and responsiveness. These findings suggest that the centromedian thalamic nuclei have an important role in initiation or propagation of generalized seizures of childhood epilepsy. In addition, complete spike-wave discharges and individual spike-wave complexes in right and left thalamic electrodes significantly preceded those in the scalp EEG at onset and during myoclonic attacks, suggesting that these types of seizure originate at or close to the centromedian thalamic nuclei region (66).
Pathophysiological considerations of the myoclonic-atonic sequence. The electrophysiological events underlying myoclonic or atonic seizures may be a consequence of genetically determined cortico-reticular hyperexcitability, which generates the generalized spike-and-wave discharges and, in turn, produces myoclonic, myoclonic-atonic, or atonic seizures depending on the predominance of inhibitory or excitatory neuronal activity as suggested by Doose (18; 17; 16).
However, it is remarkable that myoclonic-atonic seizures start in infancy and early childhood and affect children either with pre-existing cognitive or neurologic impairment as in Lennox-Gastaut syndrome or with normal neurocognitive development as in most cases of epilepsy with myoclonic-atonic seizures irrespective of etiology (see for example Lennox-Gastaut and Dravet syndrome). Hence, pathophysiology would be related to maturation and impaired functioning of brain networks rather than etiology itself (20). The correlation of myoclonus with the spike and the atonia with the slow wave is consistent with the established significance of both these events: the spike corresponds to an excitatory event, and the slow wave to an inhibitory phenomenon. Thus, the cortex and thalamus may both be involved within a loop characteristic of seizures of idiopathic generalized epilepsy, namely absences and myoclonus. In addition, the hyperexcitability determined by maturation would be counterbalanced by excess of inhibition, the combination of which would produce the sequence of jerk and atonia (20). The main difference with massive myoclonus of juvenile myoclonic epilepsy is the intensity of the jerk that also involves the lower limbs and the inhibitory component. Both could result from overexcitability of the developing cortex balanced by excess of inhibition. In one video-polygraphic study, there was a correlation between intensity of the atonia and depth of the positive component of the spike-wave complex (54).
Secondary bilateral synchrony may be the main pathophysiological mechanism in a third of patients with structural Lennox-Gastaut syndrome. Conversely, primary bilateral synchrony may be responsible in patients with genetic epilepsy with myoclonic-atonic seizures.
Secondary bilateral synchrony refers to bilateral and synchronous EEG discharges generated by a unilateral cortical focus. Contrary to secondary bilateral synchrony, primary bilateral synchrony manifests with more rapid symmetrical and synchronous generalized spike/polyspike wave discharges caused by a generalized epileptic process independent of any focal hemispheric lesion.
Study of four SLC6A1 variants associated with epilepsy with myoclonic atonic seizures showed destabilization of the protein structure (45). Knockin mice (Slc6a+/A288V) had cortical and thalamic astrocytes with reduced GABA uptake, as did human iPSC-derived astrocytes (45). Knockin mice manifested 2-4Hz spike and wave discharges (45).
The incidence of myoclonic-atonic epilepsy has been estimated to approximately 1 in 10,000 children (34). The condition is estimated to occur in 1% to 2% of all childhood epilepsies and 5.5% of children between 1 and 9 years old with a diagnosis of generalized epilepsy (17; 64). Two-thirds are boys (33).
There is no major differential diagnosis of the various epileptic syndromes with which myoclonic-atonic seizures occur. Young children may fall for other reasons than epileptic seizures; clumsiness when walking, syncope, cataplexy, vestibular disorders, and psychogenic seizures may all cause a child to fall. The epileptic nature of the falls caused by myoclonic-atonic seizures is apparent by the co-existence of other types of epileptic seizure. The primary problem in diagnosis is the differentiation of myoclonic-atonic seizures from falls as a consequence of tonic, myoclonic, or purely atonic seizures. This sometimes necessitates polygraphic video-EEG recordings for a definite diagnosis (19).
In tonic drop attacks, which are common in Lennox-Gastaut syndrome and probably exceptional in epilepsy with myoclonic-atonic seizures, falling is usually forwards with tonic flexion of the hips, upper trunk, and head as well as abduction or elevation of the arms.
Epileptic negative myoclonus, focal or generalized, is a motor symptom characterized by abrupt and brief (shorter than 500 ms) stoppage of muscular activity, not preceded by any enhancement of EMG activity. Falls may not happen as a result of negative myoclonus.
Itoh and colleagues studied with video-polygraphic recordings epileptic drop attacks in 21 children with symptomatic epilepsy of early childhood in comparison with 20 children with idiopathic myoclonic-atonic epilepsy (30). In the former, drop attacks were caused by epileptic spasms corresponding to generalized biphasic slow discharges, sharp-and-slow wave complexes, or the flattening of ongoing background activity in 15 patients; atonic seizures associated with runs of generalized spike-and-wave complexes in four patients; and myoclonic-atonic seizures in the remaining two patients. The mode of occurrence of drop attacks in epileptic spasms was periodic clustering in eight of 15 patients. Interictal EEG revealed generalized irregular multiple spikes-and-waves with focal or multifocal accentuations. Conversely, in idiopathic myoclonic-atonic epilepsy, 16 patients’ drop attacks were caused by myoclonic-atonic seizures, whereas the remaining four had myoclonic-flexor seizures, all corresponding to generalized high amplitude spikes or polyspike-and-wave complexes and occurring singly. The authors concluded that epileptic drop attacks often seen in young children with symptomatic epilepsy were most frequently caused by flexor type epileptic spasms and rarely by myoclonic-atonic seizures, a hallmark seizure type of myoclonic-atonic epilepsy. In a clinical setting, the occurrence of periodic clusters and independent focal or multifocal accentuations of generalized spike-and-wave complexes in interictal EEG may indicate epileptic drop attacks caused by epileptic spasms (30).
Epilepsy with myoclonic-atonic seizures is the prototypic disorder of myoclonic-atonic seizures that occur in all patients (09). They may also occur in Lennox-Gastaut syndrome, epilepsy with febrile seizures plus, Dravet syndrome, epileptic encephalopathy with continuous spikes and waves during sleep, and atypical partial epilepsy of childhood. The current ILAE proposed classification lists the epileptic encephalopathy with continuous spikes and waves during sleep (currently named as encephalopathy with CSWS) and the atypical partial epilepsy of childhood (currently named as atypical benign focal epilepsy of childhood) in the spectrum of developmental and/or epileptic encephalopathies with spike and wave activation in sleep (DEE-SWAS or EE-SWAS), along with the Landau-Kleffner syndrome (60).
The diagnosis of the myoclonic-atonic seizures often can be established only on the basis of video-polygraphic recording (50). However, in the context of typical features of epilepsy with myoclonic-atonic seizures, it may not be necessary to perform such a recording. The latter is mainly needed when the clinical evidence is lacking or when a major therapeutic decision is needed.
Interictal and ictal EEG. These are well described (37; 38).
At onset, the background can be normal for age. As seizure frequency increases, the interictal EEG can be slow and nearly always abnormal, reflecting the epileptic syndrome with which the myoclonic-atonic seizures occur, such as epilepsy with myoclonic-atonic seizures or Lennox-Gastaut syndrome (60). The background activity is usually slow for the average age of the subject. In addition, there may be frequent spikes in various locations and, more frequently, episodic epileptiform discharges of spike-slow waves, polyspikes, and fast rhythms. Interictal bursts of generalized 2 to 6 Hz spike and slow wave or polyspike and wave complexes, 2 to 6 seconds in duration are typical (60). The latter are usually of brief duration and asymmetrical with no apparent clinical manifestations. However, in addition to these asymptomatic discharges, there are also ictal discharges associated with a variety of seizures (tonic, atonic, myoclonic, and atypical absences).
The ictal EEG, in polygraphic video-EEG-EMG, manifests with a high amplitude 3 to 6 Hz generalized spikes and polyspikes discharge associated with the myoclonic jerk, followed by a slow wave associated with the loss of muscle tone (24; 53; 2001; 63; 52; 37; 38; 60). In myoclonic-atonic seizures associated with Lennox-Gastaut syndrome, slow spike wave complexes (less than 2.5 Hz) can be seen.
Some patients, particularly those with increased seizures before meals and cognitive impairment, should be tested for glucose transporter 1 (GLUT1) deficiency because diagnosis of GLUT1 deficiency is a strong indication for early use of the ketogenic diet, which may substantially improve the outcome of this severe disorder (48; 36; 40).
Also, some patients with cognitive and language impairment may be found to have SLC6A1 mutations (31). In a report on 116 individuals with SLC6A1 gene variants, most of which had probably loss of function mutations, epilepsy (91.1%), cognitive and developmental impairments (82.1%), and autistic traits (22.8%) were noted (26). Epilepsy with myoclonic atonic seizures was the most prevalent type, being diagnosed in 24.3% of patients (26). Additional case reports of SLC6A1 variants associated with epilepsy with myoclonic atonic seizures have been since published (67; 47).
Whole exome sequencing analysis of 101 patients with myoclonic atonic epilepsy identified pathogenic variants in 12 (14%) of 85 patients for the following genes: SYNGAP1 (synaptic ras GTPase activating protein 1), KIAA2022 (neurite extension and migration factor) , SLC6A1 (GABA transporter 1), KCNA2 (potassium voltage-gated channel subfamily A member 2), SCN2A (sodium channel 2A), STXB1 (syntaxin binding protein B1), KCNB1 (potassium voltage-gated channel subfamily B member 1), MECP2 (methyl-CpG binding protein 2), as well as ASH1L (ASH1 like histone lysine methyltransferase), CHD4 (chromodomain helicase DNA binding protein 4), and SMARCA2 (SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily A, member 2) (61).
Treatment is that of the syndrome with which these seizures occur. This is largely empirical in epilepsy with myoclonic-atonic seizures. The consensus is that valproate, which is effective in myoclonic jerks, atonic seizures, and absences, is the most efficacious of the antiseizure medications (49; 55; 35; 68; 14; 50). Add-on small doses of lamotrigine have a beneficial pharmacodynamic interaction with valproate. However, rarely valproate may induce tonic status epilepticus (27).
Topiramate reduces the frequency of atonic seizures. Ethosuximide (absences) and clonazepam (myoclonic seizures) are also beneficial. Rufinamide was found effective in a small retrospective European multicenter study on eight patients with epilepsy with myoclonic-atonic seizures (68). In retrospective small series, felbamate showed a more than 50% decrease of seizures in patients with epilepsy with myoclonic-atonic seizures (71), but because of the lack of evidence and of known side effects, no recommendations can be given for this antiseizure medication. Levetiracetam may be an effective therapeutic option, though a substantial and dose-related worsening in the frequency of the myoclonic-atonic seizures occurred in two of four patients for whom levetiracetam was used as adjunctive therapy (43). In a recent retrospective small scale study brivaracetam was also found effective (51). Add-on of sulthiame reduced seizure frequency more than 50% in 21 out of 35 patients with epilepsy with myoclonic atonic seizures (10). Complete seizure freedom was seen in 5.8%, and only mild to moderate adverse effects were reported (10).
In resistant cases, ketogenic diet is often therapeutic (08; 35; 03; 44; 56). The ketogenic diet should be started as soon as signs of epileptic encephalopathy with cognitive deterioration appear. In nonresponders to the diet and in those who do not tolerate it, the indication of ACTH or steroids should be considered. The treatment needs to be prolonged in order to prevent relapse during the period of risk, which lasts one to two years. Ketogenic diet is the treatment of choice in those 5% of patients with epilepsy with myoclonic-atonic seizures found to have glucose transporter 1 (GLUT1) deficiency (48; 36; 69). In a retrospective study of nine patients with myoclonic-atonic epilepsy, it was found that both the modified Atkins diet and ketogenic diet were efficacious in complete seizure control and allowed other medications to be stopped in seven patients (59). Two patients had greater than 90% seizure control without medications, one on the ketogenic diet and the other on the modified Atkins diet. Seizure freedom has ranged from 13 to 36 months, and during this time four patients have been fully weaned off of diet management. One patient was found to have a mutation in SLC2A1. The authors concluded that strictly defined patients with myoclonic-atonic epilepsy respond to the modified Atkins diet with prolonged seizure control. Some patients may require the ketogenic diet for seizure freedom, suggesting a common pathway of increased requirement for fats. Once controlled, those fully responsive to the diet(s) could be weaned off traditional seizure medications and in many, subsequently off the modified Atkins diet and ketogenic diet (59). In one report, a child with epilepsy with myoclonic-atonic seizures and a de novo SLC6A1 mutation had an excellent clinical response to the ketogenic diet (56). However, long-term ketogenic diet treatment stimulates liver parenchymal injury, hepatic steatosis, and gallstone formation. Therefore, patients should be monitored by screening liver enzymes and abdominal ultrasonography in order to detect these side effects (01).
In a more recent report, modified Atkins diet was applied in 30 patients with epilepsy with myoclonic-atonic seizures. By the end of the observation period, 25 (83%) of 30 patients experienced a seizure reduction of at least 50% and 14 (47%) of 30 were seizure-free (70).
Contraindicated drugs include carbamazepine, oxcarbazepine, vigabatrin, phenytoin, or phenobarbital, which increase seizure frequency in a majority of patients.
Cannabidiol-enriched cannabis has been assessed as very beneficial by parents of four children with epilepsy with myoclonic-atonic seizures (58). In June 2018, Epidiolex (cannabidiol oral formulation) from GW Pharmaceuticals obtained marketing authorization by the FDA, becoming the first drug to be approved for the treatment of Dravet syndrome in the United States market.
Early evaluation of executive functioning using both questionnaires and standardized tools is necessary for early detection of executive functioning deficit and initiating tailored rehabilitation (07).
Family support and psychotherapeutic approaches should be considered (65).
In a 2021 Delphi approach for the diagnosis and management of patients with myoclonic-atonic epilepsy, surgical approaches are worth considering in patients who have not responded to 4 to 5 antiseizure medications and ketogenic diet included vagal nerve stimulation and corpus callosotomy, the latter particularly for patients with frequent drop seizures (32).
Refer to chapters on specific epilepsy syndromes with myoclonic-atonic seizures.
All contributors' financial relationships have been reviewed and mitigated to ensure that this and every other article is free from commercial bias.
Aristea S Galanopoulou MD PhD
Dr. Galanopoulou of Albert Einstein College of Medicine received consultant fees from Synergy Medical Solutions.
See Profile
Solomon L Moshé MD
Dr. Moshé of Albert Einstein College of Medicine has no relevant financial relationships to disclose.
See ProfileNearly 3,000 illustrations, including video clips of neurologic disorders.
Every article is reviewed by our esteemed Editorial Board for accuracy and currency.
Full spectrum of neurology in 1,200 comprehensive articles.
Listen to MedLink on the go with Audio versions of each article.
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Epilepsy & Seizures
Jan. 20, 2025
Epilepsy & Seizures
Jan. 09, 2025
Epilepsy & Seizures
Jan. 09, 2025
Epilepsy & Seizures
Dec. 23, 2024
Epilepsy & Seizures
Dec. 19, 2024
Neurogenetic Disorders
Dec. 15, 2024
General Neurology
Dec. 14, 2024
General Child Neurology
Dec. 10, 2024