Epilepsy & Seizures
Tonic status epilepticus
Jan. 20, 2025
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Worddefinition
At vero eos et accusamus et iusto odio dignissimos ducimus qui blanditiis praesentium voluptatum deleniti atque corrupti quos dolores et quas.
Neuroimaging plays a critical role in diagnosing and treating adult and pediatric epilepsy. The following article details the indications for neuroimaging in patients with epilepsy and explains how neuroimaging contributes to the surgical planning for medication-resistant epilepsy. This includes discussion of structural and diffusion MRI, functional MRI, positron emission tomography, single-photon emission computerized tomography and magnetoencephalography. Automated quantitative processing of neuroimages is discussed even though these analysis methods have not yet been validated enough to become standard-of-care. The limited role of computed tomography also is discussed.
|
• All patients with medication-resistant seizures should undergo an MRI, provided there are no contraindications. | |
|
• The goal of neuroimaging in epilepsy is to identify structural or functional abnormalities that are associated with the clinically observed ictal behavior. | |
|
• Concordance of multimodal neurodiagnostic information, including ictal behavior, EEG, and neuroimaging, is necessary to localize the likely epileptogenic region. | |
|
• The role of x-ray, CT, and angiography is limited in unprovoked seizures not associated with vascular abnormalities. |
In this article, we focus on neuroimaging in adult epilepsy and present some cases that also apply to pediatrics. Epilepsy is defined as an enduring predisposition for unprovoked seizures caused by abnormally synchronous epileptiform neural activity (35). To be diagnosed with epilepsy, a patient must have had either (1) two unprovoked seizures, (2) one unprovoked seizure and evidence of a greater than 60% chance of continued seizures (discussed below), or (3) an epilepsy syndrome (23). The neuroimaging of provoked seizures is a separate topic. Further, the depth and breadth of neuroimaging findings and epilepsy syndromes of pediatric epilepsy preclude a concise discussion of both adult and pediatric epilepsy within the same topic. Similarly, discussion of the central role of electroencephalography in seizures is a separate topic.
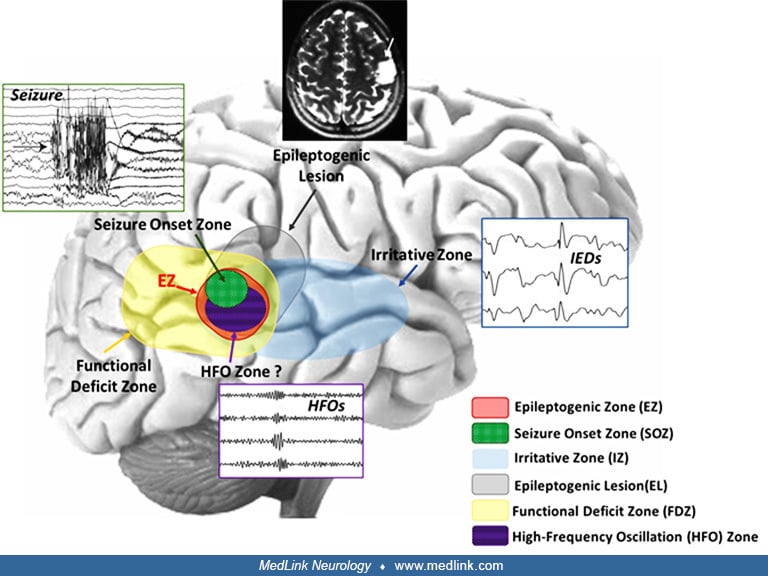
The characterization of epilepsy is based on localization of the onset and spread of epileptiform activity that cause the ictal behavior. The nomenclature of epilepsy has been revised to respect this focus on localization. Seizures are classified as either focal-onset with or without loss of awareness or generalized-onset, formerly referred to as simple or complex partial or grand mal (23). In addition, we define the following subtly different terms to more accurately describe the role of neuroimaging in localization of seizures and their onset (53):
|
• Epileptogenic lesion: a radiographically apparent abnormality that may be associated with the underlying etiology of the epileptic seizures. | |
|
• Epileptogenic zone: the cortex region that is necessary and sufficient for the generation of epileptic seizures. | |
|
• Seizure-onset zone: the cortex region where the seizure is first evident according to the diagnostic technique being used, such as scalp EEG, intracranial EEG, MEG, and ictal SPECT. | |
|
• Irritative zone: the cortex region that generates interictal epileptiform abnormality. | |
|
• Symptomatogenic zone: the first cortex region to demonstrate the first seizure manifestation, which can be either a subjective or objective manifestation. | |
|
• Functional deficit zone: the cortex region that exhibits interictal abnormal function. | |
|
• Eloquent cortex: cortex regions that are related to functions that, if lost, would result in neurologic deficits. | |
|
• Nonlesional epilepsy: epilepsy with normal neuroimaging findings, including idiopathic and cryptogenic epilepsy. Nonlesional depends on the imaging technique that is used and usually refers to conventional structural MRI-negative epilepsy without consideration of other neuroimaging modalities. |
Vollmar and Noachtar published an illustrative figure to describe the overlapping relationship between each of these zones and the information modalities used to define them (69).
The goal of resective or ablative surgery for epilepsy is to remove the epileptogenic zone while sparing eloquent cortex. Neurodiagnostic testing can provide information about the various other zones and lesions from which we infer the epileptogenic zone. However, the epileptogenic zone can only be confirmed retrospectively by removing or ablating a cortex region and rendering the patient seizure-free. The extent of this zone is theoretical because there is always the question of whether a more limited resection would also have produced seizure freedom.
The development of imaging techniques to visualize the anatomy and pathophysiology of intracranial processes has been critical to our improved understanding of the causes of seizures. In the 1930s, skull x-rays and angiograms identified aneurysms, intracranial calcifications, developmental malformations, boney lesions, and trauma that could provide indirect evidence of potential epileptogenic lesions (40). The development of tomographic imaging in the 1970s made it possible to visualize aspects of the structure of intracranial contents through x-ray CT. This made it possible to identify more subtle epileptogenic lesions, including tumors, calcifications, and gross malformations (25). The combination of tomographic imaging with radioactive tracers allowed for the visualization of neural functions through PET and SPECT. FDG-PET visualized the functional deficit zone by providing quantitative evidence of interictal hypometabolism of glucose ipsilateral to the seizure-onset zone in mesial temporal lobe epilepsy (21; 60). Similarly, when radiotracer is injected within seconds of the beginning of seizures, regions of hypermetabolism measured by SPECT can identify the seizure-onset zone.
The field of neuroimaging in epilepsy was revolutionized MRI and its ability to visualize soft tissue and, specifically, mesial temporal sclerosis associated with mesial temporal lobe epilepsy (34). As imaging techniques in MRI advanced and acquisition times decreased, MRI became able to quantify blood-oxygen-level dependent (BOLD) signal that was associated with neural activity. Although diffusion MRI plays a key role in ischemic stroke (68), its diagnostic role in epilepsy is limited.
With the various methods of analyzing the structural and functional aspects of each individual’s epilepsy, the goal is to integrate each of these clinical, neurophysiological, and neuroimaging findings into a comprehensive understanding of the patient’s epilepsy.
This section provides a brief summary of the physics behind each imaging modality and describes its function within the diagnostic assessment of a patient with epilepsy. The relative strengths, weaknesses, and contraindications for use are also reviewed. There also are excellent reviews written by others on similar topics (37; 46).
Magnetic resonance imaging (MRI). MRI is the centerpiece of neuroimaging in epilepsy because of its unparalleled ability to visualize and differentiate the soft tissues of the brain (06). Because epilepsy is a disease of neural activity, the ability to visualize subtle details in the structure and organization of neural tissue is critical to identifying a potentially epileptogenic lesion. Increasing field strength (1.5T to 3T to 7T) allows for increased contrast, increased resolution, and decreased image acquisition time. Currently, 3T imaging is standard-of-care, but 1.5T is acceptable when it is not available or contraindicated. When available, 7T imaging can provide additional details (27; 06; 28).
The physics of MRI are based on the effects of pulses radio-frequency energy that produce brief, high magnetic fields on hydrogen nuclei that have their spins aligned by a static magnetic field. This is analogous to tipping a set of spinning tops. After the magnetic pulses, hydrogen nuclei return to their prior, a lower-energy state with emission of measured radiofrequency energy. In our spinning tops analogy, the energy in the top is the angle between the top’s handle and the surface on which it spins. The rate at which the spin of these hydrogen nuclei returns to random equilibrium depends on various properties of the tissue. Based on the delay from the aligning pulse to image acquisition, T1-weighted images quantify the degree to which the spin has recovered to this random equilibrium. When the high magnetic field pulse aligned the hydrogen nuclei, it also aligns the spin of the hydrogen nuclei, which is termed in-phase. As brief time passes, small differences in the spin of the hydrogen ions from tissue properties cause them to get out of phase. In our spinning tops analogy, this is equivalent to starting all the tops spinning in the same direction from the same starting position. Over time, the spin of the tops will appear to be more and more random. The degree to which hydrogen spins are aligned is measured using T2-weighted imaging.
Based on varying pulse sequences and the time at which T1- and T2-weighted images are acquired, the MRI can generate images that are more or less sensitive to detecting differences in brain tissue.
T1-weighted images, including MPRAGE, provide the most detailed anatomical view of neural tissue. In these images, grey matter is grey, white matter is whiter, fluid is black, and fat is the whitest. These sequences are the most sensitive to visualize malformations of cortical development, including focal cortical dysplasia, encephalomalacia, and certain brain tumors. When intravenous contrast is administered, the resultant (a postcontrast) T1 image depicts a breakdown in the blood-brain barrier, which can be produced by abscesses, tumors, and infections.
T2-weighted images provide better contrast for pathologies that involve changes in water content of the tissue because fluid is bright on T2. Consequentially, white matter contains less fluid than grey matter, so it is comparatively dark. As a result of fluid sensitivity, the most prominent feature of T2-weighted images is bright ventricles. To be able to visualize periventricular pathology, the FLAIR sequence uses image acquisition techniques to suppress (darken) pure fluid signals. T2-weighted FLAIR sequences look like T2-weighted images but do not have bright CSF spaces.
Diffusion-weighted images have limited utility in nonemergent epilepsies. In status epilepticus and during or right after seizures, the region that had high metabolic activity may appear bright on diffusion-weighted images and dark on apparent diffusion coefficient through a process called restricted diffusion. The high metabolic activity causes inflammation in the tissues and locally increased cell density, thereby restricting the diffusion of hydrogen nuclei in space. This same process is why ischemic regions in stroke are bright on diffusion-weighted images and dark on apparent diffusion coefficient. This diffusion restriction can be differentiated from acute ischemic injury using perfusion imaging, which in epilepsy shows hyperperfusion compared to the contralateral side and in ischemia shows hypoperfusion.
Many centers have specialized “epilepsy protocols” for MRI in epilepsy. Based on the recommendations of the International League Against Epilepsy, these can include higher resolution anatomical scans with 1 mm3 or smaller voxels, no skipped tissue between slices, and orientation with an oblique scan along the long axis of the hippocampus (07; 06). These specialized sequences also are critical for new onset epilepsy because, in a sample of 418 participants, they identified an epilepsy-associated finding in 18%, whereas nonspecific abnormalities were observed in 17% of participants (02). When available, these are preferred to more general MRI sequences that are optimized to visualize more general pathologies and may have insufficient resolution to visualize subtle findings like hippocampal sclerosis or focal cortical dysplasias. The advantage gained from an epilepsy protocol MRI likely is higher than that gained by increased magnet strength from 1.5 Tesla to 3 Tesla (71).
Quantitative analysis of MRIs using various software techniques has been an emerging field over time, but no clear superior technique has emerged. These techniques include quantitative morphometry and volumetry with software like FreeSurfer and Morphometric Analysis Programme (MAP). MAP is a variation of voxel-based morphometry where voxel intensity and location are converted to a probability of gray matter, white matter, and CSF; this probability is compared to probabilities in a normative database (58). This technique can highlight otherwise subtle abnormalities in, for example, grey-white differentiation and transmantle signs in focal cortical dysplasia.
FreeSurfer is a software package that aligns cortical and subcortical structures using nonlinear mapping to a standard atlas and then calculates cortical thickness, regional volume, and curvature as compared to either standard databases or comparative databases (55). With the development of larger comparative databases like ENIGMA, statistical analysis techniques continue to improve (59).
These quantitative analysis techniques should be viewed as complementary to expert neuroradiologist analysis because the standardized and objective techniques highlight abnormalities that may be missed visually. There also is substantial research to develop machine-learning protocols for the identification of focal cortical dysplasia and other subtle findings in patients who otherwise would be defined as having MRI-negative epilepsy with conventional volumetry and neuroradiologist review (06; 61). Additional context can be added through expert analysis, correlating information modalities like semiology, EEG, MRI, and FDG-PET (52). Additional context can be added through expert analysis, correlating information modalities like semiology, EEG, MRI, and FDG-PET.
Positron emission tomography (PET). PET uses various radiotracers to measure function in addition to structure. In epilepsy, the most common tracer is fluorodeoxyglucose (FDG), which is a glucose analog that is taken up by high glucose utilizing tissues like gray matter. Once inside the cell, it participates in glycolysis but cannot be processed past FDG-6-phosphate; therefore, it remains in active cells before radioactive decay by emission of a positron.
PET creates images by detecting and localizing positron emissions. When radioactive decay occurs through positron emission, two positrons are emitted at 180 degrees from each other. The ring of detectors around the patient observes the coincidence of two positrons hitting the detectors 180 degrees away from each other at the same time. The resolution of PET is limited by the size of the detectors (smaller detectors correspond to a smaller region of tissue that is between two detectors) and the speed of detection (faster detectors can better identify coincident positrons and time-of-flight corrections). Noisy images occur from deflection or absorption of the positrons from their 180-degree path by interaction with tissues, which can be corrected for with a coregistered CT image. Additional noise is created by aberrant detection of positrons at detectors 180 degrees apart that happened to be from two separate sources.
The utility of interictal FDG-PET is to identify the functional deficit zone. This area has relatively low glucose metabolism between seizures compared to the neural tissue contralateral to the seizure focus. During seizures, however, the region of abnormal epileptic activity has a relatively increased metabolism. Therefore, when acquiring an FDG-PET, it is important to document acquisition time relative to the most recent seizure and consider concurrent EEG recording during acquisition to determine accurately if the scan is interictal or ictal.
Similar to a quantitative analysis of MRI, there are multiple pipelines for the quantitative analysis of FDG-PET. Especially when co-localized with other neuroimaging, these toolboxes can improve the identification of focal hypometabolism and other epilepsy-associated findings (01).
Single-photon emission computerized tomography (SPECT). Similar to PET, SPECT uses radiotracers to localize specific functions in tissue. The most common tracer used in epilepsy is technetium-99m-hexamethylpropylene amine oxime (99mTc-HMPAO), which is apparent in the brain 30 to 60 seconds after injection. The half-life requires synthesis during the morning of scanning and with required use within that day. On recognition of the seizure’s onset, the tracer is injected to visualize the areas that exhibit increased cerebral blood flow at the beginning of the seizure, thereby visualizing the seizure-onset zone. As a word of caution, a late injection can highlight the seizure-onset zone as well as spread to the rest of the network involved in seizure, resulting in less specific information.
In centers with infrastructure and experience to support quality SPECT, an ictal SPECT identified the correct site of resection in 54% of patients who were seizure-free after surgery (56). However, this low rate emphasizes that SPECT should be combined with other neurodiagnostic information. Additional considerations include the logistical aspects of being able to capture a typical seizure during the 6-hour window where the tracer was available during business hours and the associated cost of the study, especially when the patient does not have a seizure during the target time window.
Despite the use of radiotracers, the physics of SPECT imaging is more similar to x-ray CT images, but it has aspects of both. Similar to PET, SPECT relies on radioactive emission. However, instead of positron emission where coincident positrons are detected, SPECT measures a 2D picture of the number of gamma-ray photons that are emitted from a tissue. This can be thought of as analogous to a reverse x-ray where instead of passing x-rays through tissue and detecting which x-rays pass through, the tissue itself emits the gamma-rays and the detector observes these gamma-rays. Similar to x-ray CT, the 2D detector is rotated to acquire similar images at a slightly different orientation. Using reconstruction techniques identical to those used in x-ray CT, a 3D image can be constructed based on this series of 2D images.
Magnetoencephalography (MEG). The MEG signal should be thought of as perpendicular to the EEG signal. In EEG, the electrical potential difference between two points is measured to indicate the relative strengths of the electric fields. Whenever an electric field is generated, a corresponding magnetic field is generated perpendicular to the electric field. Unlike electrical potentials that can be measured using simple materials developed more than a century ago, the magnitude of the magnetic field is lower and requires superconducting interference devices (SQUIDS) to measure the small magnetic flux. The physics of this is much beyond the scope of this clinical article.
This perpendicular picture allows for MEG to provide a similar but different picture from EEG. Both modalities are critically dependent on the orientation of electrical activity: EEG is most sensitive to dipoles oriented towards or away from the surface of the brain (the peak of a gyrus or the valley of a sulcus), whereas MEG is most sensitive to perpendicular dipoles that are oriented tangentially to the surface (the cortical tissue within the fold of a sulcus). Similar to EEG, the strength of the MEG signal from neural activity reduces with the square of the distance from the source; therefore, both modalities are relatively poor at localizing signals from deep tissue.
The main benefit of MEG is the application of source modeling to localize the source of interictal discharges in 3D space (17). Sun and colleagues published an example of how raw MRI, voxel-based morphometry of MRI, FDG-PET, and MEG can be integrated to localize a lesion (64). The basic premise of this modeling is that the magnitude of interictal spikes or discharges is largest in the coils or electrodes closest to the source. If the spike is apparent on multiple coils or electrodes, the known 3D location of electrodes and knowledge of the physical properties of the tissues can be used to approximate the discharges. This provides an estimate of the irritative zone, which presumably may include the epileptogenic zone.
Importantly, this modeling requires the presence of interictal MEG spikes. Some epilepsies are not associated with the generation of interictal spikes (63). The presence of interictal spikes on EEG increases the likelihood that these MEG spikes may be apparent due to generation by neural circuits nearby in cortex but oriented perpendicular to the EEG spikes.
Although one might suppose that source analysis of raw ictal MEG may be able to localize the seizure-onset zone, this analysis has not been reliable enough in clinical application.
Functional magnetic resonance imaging (fMRI). The premise of fMRI is that subtle changes in the deoxyhemoglobin ratio to oxyhemoglobin are real-time indicators of neural activity. When a series of MR images are acquired and the timing relative to specific tasks is known, the MRI signal of the local tissue changes with respect to a hemodynamic response function. Generalized linear statistical models can be used to interpret signal changes in MRI volumetric pixels (voxels). The relative signal change in response to each task can be displayed to show which areas of eloquent cortex have a blood-oxygen level-dependent (BOLD) change in response to a task.
Specifically, fMRI is used to localize particular types of eloquent cortex, including regions necessary for generation and understanding of language and motor activity (Bohm and colleagues, fMRI activation maps for reading comprehension tasks) (10). In patients with frontal epilepsies, localization of supplementary motor areas responsible for important coordinated tasks can help guide surgical planning. For a discussion of the sensitivity of this method to identify critical language areas, see below in the Wada section.
Resting state functional MRI also has emerged as a method to measure functional connectivity based on temporal correlation of activity, with abnormalities providing additional information about localization of dysfunction (13). In resting state functional MRI, a limited resolution image is taken at a specified interval (500 ms to 5 s), and variation in each voxel over the total scan time, generally 5 to 10 minutes, is analyzed.
Intracarotid sodium amobarbital (Wada) test. The intracarotid sodium amobarbital, or Wada, test aims to lateralize function within eloquent cortex necessary for memory and language. Other than resection or ablation, Wada is seen as the gold-standard test to lateralize language and episodic memory. In original studies with postsurgical follow-up, the sensitivity and specificity for language lateralization were 98% (43/44) and 98% (45/46), respectively (11); the sensitivity and specificity for episodic memory localization was 100% (27/27) and 78% (21/27), respectively (31).
The Wada is performed through methods similar to a cerebral angiogram and is accompanied by all the risks associated with cerebral angiography, including periprocedural stroke and vascular damage. This includes a 4% risk of neurologic complications and a 0.5% to 1% risk of serious complications, including death in a patient population with the vascular risk factors typically associated with requiring a cerebral angiogram (29). In practice, these risks are considered lower in an epilepsy patient population than the vascular disease patient population used to measure the risk rates (05). For the Wada test, an intraarterial catheter is advanced into an internal carotid artery, and sodium amobarbital is injected to selectively anesthetize the anterior circulation of one cerebral hemisphere. Before and during this anesthetization, the patient’s memory and language functions are tested. A language deficit from the injection indicates the cerebral lateralization of language (language dominant hemisphere), and this can inform the likelihood of a verbal memory deficit from an anterior-mesial temporal lobe resection in that hemisphere. However, direct measurement of a memory effect of the amobarbital is critically important because of the possibility of verbal memory shifting lateralization or being diminished due to mesial temporal lobe epilepsy. If amnesia is present after one injection, an ipsilateral anterior-mesial temporal lobe resection would be considered as having a high likelihood of producing a disabling episodic memory deficit. If an injection contralateral to the anterior-mesial temporal lobe being considered for resection produces amnesia, then the test is considered as supporting dysfunction of the contralateral anterior-mesial temporal lobe (putatively epileptogenic region) and, therefore, supportive of the region’s potential of being epileptogenic.
The astute reader would note that, given the periprocedural risk of the Wada test and the relatively low risk of fMRI and MEG for patients without contraindication, the Wada would be considered obsolete. Consequentially, some centers have made the decision to stop using Wada tests and, instead, to use a combination of neuropsychological testing and language fMRI to estimate the likelihood of a postresection memory deficit.
Across multiple studies, the sensitivity of fMRI to lateralize language was only 80% to 88% (20). A larger study suggested that the positive predictive value of left lateralization by fMRI was 94% (333/356), but the predictive value of a mixed or right lateralized language on fMRI was only 50% (04). Similar protocols for lateralization of language with MEG coincide with the results of Wada testing (47; 18; 50; 24). A key limitation of fMRI and MEG is that there are no generally accepted robust methods to localize memory. Most studies focus on the concordance between fMRI/MEG and Wada for memory, and few correlate fMRI/MEG lateralization with postsurgical outcomes. In two studies that did, the fMRI only explained 60% of the variance in the postoperative memory function (49; 41).
X-ray computed tomography. Due to the factors discussed above, CT is relatively insensitive to the detection of the subtle cortical abnormalities that are associated with epilepsy.
The main function of CT is to identify intracerebral hemorrhage, calcifications, large tumors, and gross structural abnormalities like hydrocephalus. By the definition of epileptic seizures as unprovoked, seizures caused by acute intracerebral hemorrhage are not epilepsy because they are provoked. Epilepsy that develops after an intracranial hemorrhage occurs later due to chronic changes caused by the hemorrhage. Calcifications can suggest vascular abnormalities, infections, or consequences of infections, all of which can provoke seizures; this can be seen in figure 1 of the article by Zhao and colleagues (70). Lastly, hydrocephalus can reflect underlying gross cortical malformations, but seizures acutely provoked by intracranial hypertension or complications of intracranial hypertension are not considered epilepsy.
The physics of CT imaging is no more complex than x-ray imaging: a source of x-rays is placed on one side of tissue, and a detector is placed on the other side. The degree to which x-rays are detected reflects the amount of x-ray absorption between the source and detector and is roughly related to tissue density. Individual 2D images acquired from a specific source to detector are acquired with varying orientation relative to the tissue. Using reconstruction techniques, a 3D approximation of the tissue density can be estimated. The main limitation in cranial CT is beam hardening artifact where particularly dense tissues absorb almost all x-rays. Therefore, it is difficult to estimate the degree to which tissue distal from the source absorbs x-rays. For tissue that is completely encircled by dense bony tissue like the cerebrum, this results in poor image contrast. For cerebellar and brainstem tissue that is tightly encircled by bones, the reconstructed image is almost useless clinically except for detecting gross pathology. When MRI is not available due to resource limitations or contraindications, however, CT may provide the best available structural image.
Other neuroimaging modalities. The following neuroimaging modalities have limited use in the diagnosis and treatment of clinical epilepsies but have had positive impacts on research. Simultaneous EEG and fMRI have been used to validate EEG source localization and has inspected the hemodynamic correlates of epileptiform spikes and nonmotor seizures (19). Diffusion-weighted MRI and tractography can be used to trace major connections between the eloquent cortex, specifically between Broca’s and Wernicke’s areas. Anatomic localization of these pathways can help surgical planning by preserving white matter pathways that are necessary for language but are poorly identified by fMRI. This technique, however, is rarely implemented in practice. Lastly, in the past decade, there was great excitement in the development of magnetic resonance spectroscopy wherein instead of focusing on the spin of hydrogen ions, imaging is based on atoms with higher resonances. The image acquisition time, poor signal-to-noise ratio, and poor spatial localization of signals have limited the application of MRS to epilepsy.
Therefore, neuroimaging should always be combined with clinical history and EEG to localize the seizures. Neuroimaging abnormalities, especially those that do not colocalize with ictal behavior or ictal electrographic findings, may not be epileptogenic despite the extent of their abnormality.
One of the main functions of neuroimaging in epilepsy is the identification of focal epileptogenic lesions, even when ictal behavior may appear to have a generalized onset. The coregistration of MRI to FDG-PET can improve the identification of subtle epileptogenic lesions on MRI by using relative hypometabolism to identify regions for more detailed inspection (54; 36). Another emerging function of neuroimaging is to identify biomarkers of epileptogenesis, defined by the future development of new-onset epilepsy (16).
Mesial temporal lobe epilepsy. A common type of adult epilepsy is mesial temporal lobe epilepsy, which is associated with complex febrile seizures or febrile status in childhood (57). Complex febrile seizures are defined by having a duration longer than 15 minutes but less than 30 minutes, recurrent febrile seizures in a 24-hour period, or focal febrile seizures. Febrile status epilepticus involves febrile seizures longer than 30 minutes.
The MRI features of mesial temporal sclerosis are reduced hippocampal volume, increased T2-weighted intensity, decreased T1-weghted intensity, and loss of typical hippocampal structure, including reduced grey-white differentiation and asymmetry of lateral ventricular horns. This also can include atrophy or asymmetry of the ipsilateral fornix and mammillary bodies. Pathologically, this typically includes focal neuronal cell loss in CA1 but can include or be focused in other regions (09).
Although numerous research applications have shown that automated volumetry can identify hippocampal sclerosis, these automated methods have not been shown to be as reliable as neuroradiologist interpretation when the neuroradiologist has experience in epilepsy. The main challenge is the application to clinical quality images from patients that may have hippocampal and extrahippocampal structural deficits that cause artifacts in automated segmentation. With interictal FDG-PET, hypometabolism ipsilateral to the sclerotic hippocampus is present.
Focal cortical dysplasia. Focal cortical dysplasia is a histologic diagnosis that can be made and subtyped after tissue is available for microscopy. MRI features that suggest focal cortical dysplasia include blurring of the grey-white junction, focal thickening of grey matter, abnormal gyration and sulcation, thickening at the deep sulci, and the transmantle sign (tapering of white matter signal from cortex to ventricle).
Focal cortical dysplasias can be split into subtypes based on the underlying neurohistopathology as well as neuroimaging according to the International League Against Epilepsy (ILAE) consensus (08; 44; 43).
Focal cortical dysplasia type 1. Type 1 is evident as prominent segmental or lobar atrophy or hypoplasia with loss of regional white matter volume, typically seen with moderately increased T2/FLAIR hyperintensity and decreased T1 signal, with moderate blurring of the grey-white junction. Type 1a is typically temporal, whereas 1b is extratemporal; temporal focal cortical dysplasia associated with hippocampal sclerosis is type 3a.
Focal cortical dysplasia type 2. Type 2 is evident as abnormal gyri and sulci, thickened cortex, and marked blurring of the grey-white junction. Type 2a contains only dysmorphic neurons, whereas 2b also contains balloon cells. The blurring of the grey-white junction may extend from cortex to ventricle as in the transmantle sign. These MRI features are more likely in frontal lobes than temporal lobes when compared to Type 1 focal cortical dysplasia.
Focal cortical dysplasia type 3. Type 3 is a focal cortical dysplasia associated with another abnormality, including hippocampal sclerosis (3a), glioneuronal tumor (3b), vascular malformation (3c), or early cerebral developmental abnormality (3d) (38).
Tuberous sclerosis. Tuberous sclerosis is a special class of malformations of cortical development that includes a syndrome of noncancerous tumors of tissues derived from the ectodermal developmental layer including brain, skin, kidneys, and other organs.
MRI evidence of tubers includes subcortical or cortical regions with high T2 and low T1 signal, sometimes with enhancement or calcification; subependymal nodules (enhance and often show calcification, high T1 and isointense to high T2); subependymal giant cell tumors (SGCT, intensely enhancing); white matter abnormalities, and other rarer findings. In patients with multiple intracranial tubers, FDG-PET and other functional imaging modalities can localize a single epileptogenic tuber in some patients (14).
Sturge-Weber syndrome (vascular phakomatosis). The classic findings of Sturge-Weber Syndrome on MRI were enhancing leptomeningeal angiomatosis and calcifications (48). The pattern of calcification and magnetic susceptibility artifact on T2* has been described as “tram-tracks.” These abnormalities frequently were associated with abnormal gyration of tissue.
Nodular heterotopias. Nodular heterotopias are ovoid appearing masses of grey matter that have not completed the process of migration to the appropriate cortical layer. Most commonly, these are periventricular, but they also can be seen in subcortical bands. Although the nodules themselves can generate seizures, they represent an abnormality of migration; therefore, the overlying areas of cortex often are abnormal, including focal cortical dysplasias.
Other malformations of cortical development. Malformations of cortical development associated with epilepsy include but are not limited to encephaloceles, ulegyria, polymicrogyria, heterotopias, hemimegalencephaly, schizencephaly, lissencephaly (figure available here) (66), lissencephaly (figure available here) (62), and other findings. Multiple types of abnormalities can be seen within single syndromes like neurofibromatosis type 1 (figure available here) (03).
When large volume cortical malformations are present, non-CT or MRI modalities may be able to identify the portion of the malformation that may be epileptogenic. This can include focal hypometabolism on FDG-PET or interictal magnetic spike localization with MEG.
Hypothalamic hamartoma. In individuals with gelastic seizures, one should suspect hypothalamic hamartomas. Imaging features include suprasellar masses that are hypointense on T1 and slightly hyperintense on T2 and FLAIR and do not enhance after contrast.
Neurocysticercosis. Infectious etiologies for seizures may initially appear as seemingly unprovoked seizures. In neurocysticercosis, seizures are provoked by death of the parasite and resulting immunologic irritation of tissue. While the parasite is alive, it forms a hypodense nonenhancing cyst. As the parasite develops, this cyst can enhance on CT and MRI and have associated edema and eventually calcification.
Toxoplasmosis. Neurotoxoplasmosis most commonly occurs in immunocompromised patients and resembles the findings of an intracerebral abscess: hypodense on CT and variably intense on T1 and T2-weighted MRI but with consistent ring or nodular contrast enhancement on both CT and MRI.
Vascular-related epilepsies. Seizures in vascular-related epilepsies are due to the irritative effect of blood on neural tissue. If seizures are purely due to this irritative effect, then technically the patient has regular seizures provoked by intracerebral (micro)hemorrhage. By the definition of epilepsy as an enduring predisposition for unprovoked seizures, there are some who believe that vascular-related seizures are not a subtype of epilepsy. Instead, they are a subtype of recurrent seizures. However, differentiating recurrent microhemorrhages from hemorrhage-produced enduring abnormality is unlikely to be possible, and the risk of recurrent hemorrhage cannot always be treated; therefore, many patients are treated with long-term antiseizure medications.
There are a number of vascular-related epilepsies, including cerebral amyloid angiopathy, cerebral cavernous malformations, and arteriovenous malformations. When the intracranial blood involves cortex, the patient is more likely to develop seizures (33). Not all patients with vascular abnormalities on imaging develop seizures. Cerebral amyloid angiopathy is associated with numerous microhemorrhages in older individuals (figure available here) (15). Cerebral cavernous malformations have a “popcorn” appearance on MRI and have a much higher risk of epilepsy, with 94% of patients developing recurrent seizures within 5 years of diagnosis (32); figure available here and also in articles by Maehara and Rigamonti (39; 51; 30). The risk of epilepsy is much lower in patients with arteriovenous malformations and is estimated at 58% (32); for example images, see article by Ollivier and colleagues (45).
Tumor-associated epilepsies. Epilepsy can be a presenting sign or associated finding in multiple intracranial tumors. The general pathophysiologic seizure mechanism is inflammatory irritation or effacement of normal neural tissue resulting in hyperexcitability or dysregulation of normal neural networks. Intracranial tumors that are associated with seizures include dysembryoplastic neuroepithelial tumor (DNET); multinodular and vacuolating neuronal tumor (MVNT); gliomas, of various types, including glioblastoma multiforme (GBM); metastatic lesions; meningiomas; and lymphomas (67).
Each of these intracranial tumors has varying findings on neuroimaging (38).
Dysembryoplastic neuroepithelial tumors have been described as “bubbly” with multiple lobulated regions of T2 hyperintensity and calcifications that can be apparent on CT and T2* sequences. Multinodular and vacuolating neuronal tumors include multiple ovoid nodules at the junction of superficial subcortical white matter and a deep cortical ribbon, often surrounding a sulcus. Unfortunately, these nodules associated with multinodular and vacuolating neuronal tumors also can be described as “bubbly.” The neuroimaging findings of gliomas and other intracranial neoplasms are well summarized elsewhere in Medlink.
Encephalomalacia. Encephalomalacia is a nonspecific term describing physical malformation of the brain that often includes tissue loss and is related to an injury. Alteration of brain networks through injury can be epileptogenic, but there is incomplete understanding of why some patients with encephalomalacia develop seizures, some medication-refractory, and others do not.
Autoimmune epilepsies. The identification of autoimmune or paraneoplastic seizures includes both clinical history, cerebrospinal fluid analysis, neuroimaging, and, potentially, other diagnostic evaluations (12). As new antibodies are identified, this field continues to develop. In their articles, Budhram and colleagues and Guerin and colleagues highlight some of the typical findings associated with each of the common antibodies (12; 26).
There is substantial overlap between the imaging features of each antibody-associated encephalitis; therefore, it is appropriate to send a panel of antibodies when autoimmune encephalitis is suspected. It also is important to differentiate these autoimmune epilepsies from common mimics; for example, mimics, see the article by Budhram and colleagues (12).
Rasmussen encephalitis. Rasmussen encephalitis is a severe, progressive, likely immune-mediated encephalitis that typically involves most or all of one hemisphere. Patients typically are children and have frequent seizures and status epilepticus, often needing hemispherectomy despite immunomodulatory therapy (eg, steroids). Imaging can be variable and reflects the stage of the disease, with profound diffusion restriction after status epilepticus, T2 hyperintensities associated with edema, followed by long-term atrophy and gliosis.
The following flowchart summarizes the neuroimaging component of the diagnosis and assessment of patients for epilepsy surgery at one center where FDG-PET is available.
For new-onset seizures, status epilepticus, or seizures that differ from the patient’s typical seizures, neuroimaging can be used in the diagnostic assessment. This should be tailored based on the patient’s history to address the most likely, most dangerous, and most time-critical. This may be limited to iodinated contrast-enhanced CT in the context of acute stroke or may include MRI epilepsy protocol with and without gadolinium contrast when intracranial tumors like glioblastoma are suspected.
For the assessment of newly diagnosed epilepsy, high-resolution anatomical imaging is indicated to determine if the seizures may be caused by an intracranial tumor, vascular abnormality, intracerebral infection, or malformation of cortical development. In the absence of contraindications, the standard of care for this is a 3T MRI with an epilepsy-specific sequence protocol described by Bernasconi and Bernasconi (06).
For the presurgical assessment of medication-resistant epilepsy, neuroimaging plays a key role in identifying a single, apparently epileptogenic zone that may be resected. At our center, the first stages of assessment include MRI and FDG-PET imaging in combination with video-electroencephalography. If ictal behavior, electrophysiology, and neuroimaging all identify a single, resectable focus, then the patient may be able to skip intracranial electrographic monitoring. In the case of insufficient concordance, MEG and SPECT can be used to build evidence for a single resectable region prior to intracranial EEG. If the patient is not a candidate for a resective or ablative surgery, they may be a candidate for neuromodulatory treatments (see the article on brain stimulation for epilepsy). Lastly, once a hypothesized epileptogenic zone is identified, neuropsychological testing, fMRI, MEG, and Wada testing, can contribute to the decision if a resection is likely to produce a functional deficit.
All contributors' financial relationships have been reviewed and mitigated to ensure that this and every other article is free from commercial bias.

Wesley T Kerr MD PhD
Dr. Kerr of University of Pittsburgh School of Medicine received consulting fees from Biohaven Pharmaceuticals and SK Lifesciences.
See Profile
John M Stern MD
Dr. Stern, Director of the Epilepsy Clinical Program at the University of California in Los Angeles, received honorariums from Ceribell, Jazz, LivaNova, Neurelis, SK Life Sciences, Sunovian, and UCB Pharma as advisor and/or lecturer.
See ProfileNearly 3,000 illustrations, including video clips of neurologic disorders.
Every article is reviewed by our esteemed Editorial Board for accuracy and currency.
Full spectrum of neurology in 1,200 comprehensive articles.
Listen to MedLink on the go with Audio versions of each article.
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Epilepsy & Seizures
Jan. 20, 2025
General Neurology
Jan. 13, 2025
General Neurology
Jan. 13, 2025
Epilepsy & Seizures
Jan. 09, 2025
Epilepsy & Seizures
Jan. 09, 2025
Neuro-Ophthalmology & Neuro-Otology
Jan. 08, 2025
Neuro-Ophthalmology & Neuro-Otology
Jan. 07, 2025
General Neurology
Dec. 30, 2024