General Neurology
Use of focused ultrasound in neurologic disorders
Jan. 13, 2025
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Worddefinition
At vero eos et accusamus et iusto odio dignissimos ducimus qui blanditiis praesentium voluptatum deleniti atque corrupti quos dolores et quas.
This article describes the neurologic effects of various biological agents that could be used in biowarfare and bioterrorism. Clinical manifestations and pathophysiology of the important agents from a neurologic perspective (particularly anthrax and botulism) are described.
|
• Anthrax has been used as a biowarfare and bioterrorism agent, and repeated (though unsuccessful) attempts have been made to use botulinum toxin as a bioterrorism agent. | |
|
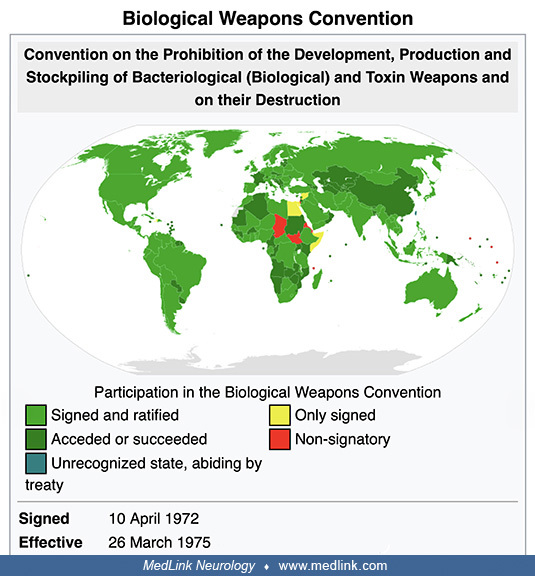
• The Biological Weapons Convention, entered into force in 1975, prohibits the development, production, acquisition, transfer, stockpiling, and use of biological and toxin weapons. | |
|
• Severe hemorrhagic meningitis regularly occurs in anthrax and is often accompanied by severe systemic disease, most likely transmitted by spore inhalation. | |
|
• Neurologists are most likely to become involved in primarily diagnosing bioterrorist attacks that use botulinum toxin through oral ingestion or inhalation. | |
|
• Characteristics that make a pathogen a high risk for bioterrorism include a low infective dose, ability to be aerosolized, high contagiousness, and hardiness (ie, survival in various environmental conditions). | |
|
• In the CDC 3-tier categorization of bioterrorism agents and diseases, Category A is comprised of high-priority agents that pose a risk to national security because (1) they can be easily disseminated or transmitted from person to person, (2) they have the potential for major public health impact (eg, high case-fatality rates), (3) they will likely cause public panic and social disruption, and (4) they require special measures for public health preparedness. | |
|
• Diagnostic and management recommendations for anthrax meningitis and botulism vary across conventional (noncrisis), contingency, and crisis settings. |
Biological weapons and bioterrorism agents include replicating agents (eg, bacteria, spores, viruses) and nonreplicating agents (eg, toxins).
Individuals in many cultures have employed biological agents for execution, assassination, and murder. However, many of the claims concerning the use of biological weapons in ancient history are not supported by rigorous evidence and, although seemingly cited in the introductions to numerous articles, editorials, and monographs on bioweapons and bioterrorism, are unreferenced and uncritical rehashing of speculative and dubious claims, often reiterated and amplified on social media (56). Supported and plausible claims include reported attempts to poison water supplies with diseased carcasses (eg, the hurling of plague- or smallpox-infested material or bodies across protective barriers) and deliberate attempts to spread smallpox in enemy armies or populations using biologically inoculated fabrics and people.
The Geneva Protocol (1925): "no use" versus "no first use" of biological weapons. The Geneva Protocol (formally known as the Protocol for the Prohibition of the Use in War of Asphyxiating, Poisonous or other Gases, and of Bacteriological Methods of Warfare) was signed in Geneva in June 1925 and entered into force in February 1928. The Geneva Protocol prohibits the use of biological weapons, a first important milestone towards a comprehensive ban on biological weapons. Several States ratified the Protocol with reservations concerning the Protocol’s applicability and regarding the use of chemical or biological weapons in retaliation, effectively rendering the Geneva Protocol a "no-first-use agreement."
|
The Undersigned Plenipotentiaries, in the name of their respective Governments: ... Declare: ... That the High Contracting Parties, so far as they are not already Parties to Treaties prohibiting such use ... agree to extend this prohibition [on the use in war of asphyxiating, poisonous or other gases, and of all analogous liquids, materials or devices] to the use of bacteriological methods of warfare and agree to be bound as between themselves according to the terms of this declaration (Protocol for the Prohibition of the Use in War of Asphyxiating, Poisonous or Other Gases, and of Bacteriological Methods of Warfare. Accessed October 26, 2022). |
Bioterrorism and "neuroterrorism." Bioterrorism is defined as the intentional use of biological, chemical, nuclear, or radiological agents to cause disease, death, or environmental damage. Bioterrorism that intentionally targets the nervous system has been labeled “neuroterrorism” (23).
Because civilian panic and disruption of institutional operations are prominent intentions (and outcomes) of terrorist attacks, panic and psychologically determined “me-too” symptomatology will contribute significantly to the diagnostic and management burden (59).
History of the United States Biological Warfare Program. The United States did not begin an offensive biological warfare program until 1941, when concern about the German and Japanese biological warfare threats motivated the United States to begin biological weapon development (150).
During World War II, Allied intelligence services suspected the German military might use botulinum toxin as a biological weapon, especially during preparations for Operation Overlord, the Allied invasion to liberate Europe (150). However, despite these persistent concerns, botulinum toxin was not part of the German military arsenal (even if some German scientists had tried to use French pre-war military research for this purpose). The misinformation spread by British and American intelligence services stimulated military biological weapons research at the Porton Down facilities in England and at Camp Detrick in the United States.
During the next 28 years, the United States initiative evolved into a military-driven research and acquisition program shrouded in controversy and secrecy. Most research and development was done at Fort Detrick, Maryland, whereas production and testing occurred at Pine Bluff, Arkansas, and Dugway Proving Ground in Utah. Field testing was done secretly with simulants and actual agents disseminated over wide areas.
Initially, a small defensive effort paralleled the weapons development and production program. Later, the program became entirely defensive with the decision of President Richard Nixon in 1969 to halt offensive biological weapons production and the subsequent agreement in 1972 at the international Biological Weapons Convention never to develop, produce, stockpile, or retain biological agents or toxins. The U.S. Biological Defense Research Program now conducts research to develop physical and medical countermeasures to protect service members and civilians from the threat of biological warfare.
Prior "simulated germ-warfare attack" by the U.S. military: Operation Sea-Spray (1950). Operation Sea-Spray was a secret U.S. Navy biological warfare experiment conducted in September 1950. In the experiment, Serratia marcescens and Bacillus globigii bacteria were sprayed over the San Francisco Bay Area in California to assess the vulnerability of a city like San Francisco to a bioweapon attack (46; 145). From September 20th until September 27th, the Navy used giant hoses to spray a fog of bacteria (species then believed to be harmless) from a ship cruising off the shore of San Francisco. Based on results from monitoring equipment at 43 locations around the city, the Army determined that nearly all the city's 800,000 residents had inhaled at least 5,000 bacteria. Because the military believed the organisms were harmless, no adequate epidemiological studies were done at the time to assess health outcomes of the experiment, performed without the consent of the city's residents. However, unusual collections of urinary tract infections and pneumonia were identified at the time.
Over the next 20 years, the U.S. military conducted 239 "germ-warfare" tests in populated areas, according to news reports and congressional testimony (46; 145). Tests included the large-scale releases of bacteria in Minneapolis, St. Louis, the New York City subway system, on the Pennsylvania Turnpike, and in the National Airport (ie, now Ronald Reagan Washington National Airport in Arlington County, Virginia across the Potomac River from Washington, D.C.).
The Biological Weapons Convention (1972). Disarmament talks after World War II originally addressed biological and chemical weapons together but remained inconclusive. Shortly after states finalized negotiations on the Nuclear Non-Proliferation Treaty in 1968, an initiative in the United Kingdom broke the impasse in discussions of chemical and biological weapons: the UK proposed (1) to separate consideration of biological and chemical weapons and (2) to concentrate first on the former. From 1969 until 1971, the Biological Weapons Convention (BWC) was negotiated in Geneva, Switzerland, within the Eighteen Nation Committee on Disarmament (ENDC) and the Conference of the Committee on Disarmament (CCD).
Formally known as “The Convention on the Prohibition of the Development, Production, and Stockpiling of Bacteriological (Biological) and Toxin Weapons and on their Destruction,” the BWC opened for signature in 1972 and entered into force in March 1975.
The BWC prohibits developing, producing, acquiring, transferring, stockpiling, and using biological and toxin weapons. It was the first multilateral disarmament treaty banning an entire category of weapons of mass destruction. The BWC supplements the 1925 Geneva Protocol, which prohibited only the use of biological weapons.
|
Convention on the Prohibition of the Development, Production, and Stockpiling of Bacteriological (Biological) and Toxin Weapons and on Their Destruction ... Article I. Each State Party to this Convention undertakes never in any circumstances to develop, produce, stockpile, or otherwise acquire or retain: (1) microbial or other biological agents, or toxins whatever their origin or method of production, of types and in quantities that have no justification for prophylactic, protective, or other peaceful purposes; (2) weapons, equipment, or means of delivery designed to use such agents or toxins for hostile purposes or in armed conflict. (Convention On The Prohibition Of The Development, Production And Stockpiling Of Bacteriological (Biological) And Toxin Weapons And On Their Destruction. Accessed October 26, 2022.) |
Since 1980, States Parties have met approximately every 5 years to review the operation of the Biological Weapons Convention. The BWC has reached almost universal membership with 184 States Parties and four Signatory States (Convention on the Prohibition of the Development, Production and Stockpiling of Bacteriological (Biological) and Toxin Weapons and on Their Destruction. Accessed October 26, 2022). Unfortunately, the effectiveness of the BWC has been limited by insufficient institutional support and the absence of any formal verification process to monitor compliance.
Photograph from U.S. Mission by Eric Bridiers on January 24, 2012. The United States Mission to the United Nations and Other International Organizations in Geneva represents the United States government in Geneva. (Creative Com...
The 1984 Rajneeshee bioterrorism attack. In 1984, 751 people became ill with salmonellosis food poisoning in The Dalles, Oregon, due to deliberate Salmonella contamination of salad bars at 10 local restaurants. A group of prominent followers of Bhagwan Shree Rajneesh (later known as Osho, leader of the Rajneeshee cult), had hoped to incapacitate the voting population of the city so that their own candidates would win the 1984 Wasco County, Oregon, elections. The incident was the first and remains the single largest bioterrorist attack in United States history. The simplicity of the dissemination and its apparent effectiveness stimulated efforts by other terrorist groups with biological and chemical weapons.
Four of the restaurants in The Dalles, Oregon, affected by the Rajneeshee cult bioterrorism incident in 1984. (Source: Wikimedia Commons. User: Cacophony [2008]. Creative Commons Attribution 3.0 Unported [CC BY 3.0] license, cr...
The salsa bar of The Dalles' Taco Time that had been intentionally contaminated as part of the Rajneeshee cult bioterrorism attack in 1983 in The Dalles, Oregon. (Source: Wikimedia Commons. User: Cacophony [2008]. Creative Comm...
Prior use of anthrax as biological warfare agent. Anthrax has been used on a limited basis as both biological warfare and a bioterrorism agent.
During World War I, German agents reportedly tried to infect allied horses as they were being shipped to the European front (47; 166), although other accounts discount this claim (139). Japan reportedly first developed anthrax as a bioweapon in the 1930s and used anthrax against China during World War II (139; 47; 69; 166; 154). In 1993, the Japanese terrorist group Aum Shinrikyo dispersed aerosols of anthrax and botulism throughout Tokyo on at least eight occasions (76; 77), but these attacks failed to produce illness, apparently in part because the terrorists mistakenly used a harmless anthrax strain developed for vaccines (127). In 2001, anthrax was used as a bioterrorism agent in the United States, with several deaths, more than 20 cases, and over 32,000 individuals receiving postexposure prophylaxis because of anthrax delivered through the United States mail system (27).
The United States began researching offensive bioweapons in 1943 at Camp Detrick (now Fort Detrick) in Frederick, Maryland. To assess the risk of covert biological attacks in the 1960s, the army conducted large-scale covert tests at various civilian sites (eg, National Airport and Greyhound Terminal in Washington, D.C., and the New York City subway), using the anthrax simulant Bacillus globigii (139). The United States bioweapons program, which included the development of weaponized anthrax, was terminated in 1969 following an executive order by President Richard Nixon. All stockpiles of biological agents were destroyed by May 1972 (154). There is no evidence that these U.S. weapons were ever deployed.
In 1972, the United States, the United Kingdom, and the USSR signed the Convention on the Prohibition of the Development, Production, and Stockpiling of Bacteriological (Biological) and Toxin Weapons and on Their Destruction ("The Biological Weapons Convention") (154). This treaty prohibits the research, development, and stockpiling of biological agents for offensive military purposes. Although this biological weapons treaty has since been ratified by more than 140 countries, biological warfare research and the development of biological weapons have continued in many countries (154).
The Soviet bioweapons program began before World War II under the auspices of Biopreparat, their bioweapons agency. This program continued until the 1990s despite the Biological Weapons Convention. By the 1980s, Biopreparat could produce thousands of tons of weaponized anthrax annually. Biopreparat also developed antibiotic-resistant anthrax using recombinant DNA techniques.
In April 1979, an accidental release of anthrax spores occurred at a military bioweapons factory (Military Compound 19) in Sverdlovsk in the former Soviet Union (now Yekaterinburg, Russia) (01; 01b; 104; 79; 47; 163; 154). This incident resulted in at least 66 human deaths (among the 77 patients identified) in a narrow zone up to 4 kilometers downwind from the facility, as well as outbreaks of anthrax in livestock up to 50 kilometers downwind (104). Although the Soviet Ministry of Health initially blamed the deaths on cutaneous and gastrointestinal anthrax occurring from the consumption of contaminated meat, this scenario was doubted by military sources in the West (54; 111; 01; 104; 79; 47; 154). In 1992, Russian President Boris Yeltsin confirmed that this outbreak was a result of "military developments" (104), and clinical and epidemiologic studies documented inhalational anthrax from a mixture of different Bacillus anthracis strains as the cause (01; 104; 79).
In the aftermath of the Gulf War, Iraq acknowledged to the United Nations Special Commission Team 7 that it had conducted biological weapons research with various agents, including Bacillus anthracis (154). Further details were uncovered in 1995. Iraq had extensive research facilities at multiple sites, including Salman Park on the Tigris River. Iraq conducted field trials with Bacillus subtilis (an anthrax simulant) and various biowarfare agents using various delivery systems, including rockets, aerial bombs, sprayers attached to helicopters, and possibly unmanned drones (154). To reduce the particle size to maximize the delivered dose of anthrax, Iraq used sequential filters in an arrangement reportedly like that used at Camp Detrick in the 1950s. Iraq produced 8500 liters of concentrated anthrax, of which 6500 liters were placed into R400 bombs, Al Hussein warheads, and other devices (154).
Anthrax has been developed as a biological warfare agent by at least seven countries. It remains a significant bioweapons threat (53) and is considered the most likely biological warfare agent, as it is stable in spore form and can be stored for prolonged periods, it is easy and cheap to produce, there is no natural immunity in industrialized nations, it can be dispersed in air, the inhalational form is highly lethal, and the agent is difficult to detect (155). Nevertheless, aerosolized anthrax has not yet been used on the battlefield, possibly in part because of the “moral repugnance” of biological weapons, potential retaliation in kind, delayed manifestations following use, and uncertain effects depending on weather conditions (128). Indeed, the uncertainties of such weapons are demonstrated by the Sverdlovsk incident in the USSR in 1979, which involved the accidental release of an estimated 10 kilograms of weapons-grade anthrax spores: this accident produced a total of only 66 fatalities among a potentially exposed population of 1.2 million people (128).
Offensive anthrax bioweapons were initially manufactured as slurries of highly concentrated bacteria. Although easy and safe to manufacture, such slurries had to be refrigerated for storage and were difficult to disseminate (47). Later, freeze-dried powder formulations of anthrax spores were developed as bioweapons that, although technically difficult and dangerous to manufacture, were thermally stable without refrigeration and were easily disseminated (47). Bioweapon anthrax may also be modified to be multidrug-resistant and may be stabilized with other substances (eg, silica) to decrease electrostatic charges imparted by the milling process and, thereby, keep the particles from clumping. The latter issue is critical because biowarfare agents are most effectively delivered as an aerosol of particles from 1 to 5 microns in size (47). Particulates in this size range behave similarly to a gas and are taken into bronchioles and alveoli during respiration, whereas larger particles are much more difficult to disperse, rapidly fall to the ground, or become trapped in the upper airway (47). Anthrax aerosols are invisible, odorless, colorless, and tasteless.
Prior use of anthrax in bioterrorism. In late 2001, an anthrax outbreak attributed to bioterrorism occurred in the United States. Anthrax was spread through the mail (29; 30). Twenty-two cases were identified, 11 with inhalational anthrax and 11 with cutaneous anthrax (30; 31; 32; 33; 81; 78; 67). Five of the inhalational cases died. Only one of these cases had documented anthrax meningoencephalitis (81).
The FBI alleges that the 2001 anthrax bioterrorism outbreak in the United States was conducted by U.S. Army biodefense scientist Bruce Ivins, who worked at the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID) in Fort Dettrick, Maryland (106; 22). In 2008, shortly before these allegations were made public, Ivins committed suicide. The FBI employed a complex strategy to identify the source of the anthrax: (1) spores from the mailed envelopes were cultured, yielding thousands of colonies, one from each spore; (2) a small number of colonies with unusual features (“minority phenotypes”) were identified, and the genomes of these colonies were completely sequenced to identify the corresponding mutations; (3) tests were developed to screen anthrax samples for four of these mutations, using a polymerase chain reaction-based strategy; these molecular tests were applied to more than 1000 isolates from labs in the United States and other countries (62). In only eight of the study’s samples were all four mutations identified, and all were directly related to spores Ivins had created in 1997 (62). However, in February 2011, a scientific panel of the U.S. National Academy of Sciences independently evaluated the genetic evidence and concluded that it was insufficient to prove that Ivins was responsible. In addition, Ivins's laboratory did not contain the equipment needed to manufacture the refined powder of spores that were implicated in the 2001 bioterrorism attacks (22).
Anthrax is now classified as a category A biological warfare agent, a category of microorganisms or toxins that can be easily spread, leading to intoxication with potentially high death rates (08).
Prior attempts to use botulinum toxin in biowarfare and bioterrorism. Botulinum toxin has been considered by multiple countries as a potential bioweapons agent, with a possible attempt to use botulinum toxin by supporters of Francisco ("Pancho") Villa (1878-1923) against Mexican federal troops in 1910.
During World War II, the Nazis reportedly developed botulinum toxin as a bioweapons agent. The United States and the Soviets also explored botulinum toxin as a potential bioweapon, both ultimately dismissing botulinum toxin as inferior to other agents such as anthrax and tularemia.
Saddam Hussein (1937-2006) began an extensive biological weapons program in Iraq in the early 1980s despite having signed (though not ratified until 1991) the Biological Weapons Convention (1972). Details of the Iraq bioweapons program surfaced after the Gulf War (1990-91) during the disarmament of Iraq under the United Nations Special Commission (UNSCOM). By the end of the war, Iraqi scientists had investigated the bioweapons potential of five bacterial strains, one fungal strain, five types of virus, and four toxins; of these, anthrax, botulinum toxin, and aflatoxin had proceeded to weaponization for possible deployment. Iraq claimed it had produced 19,000 liters of the toxin, sufficient (if true) to kill the world's entire human population. A vial of Clostridium botulinum bacteria was found by investigators in Iraq after the ouster of Hussein, leading to much greater public attention on the potential of botulinum toxin as a bioweapon.
In the 1990s, the Japanese doomsday cult Aum Shinrikyo produced botulinum toxin and spread it as an aerosol in downtown Tokyo, but the attacks caused no fatalities (95; 05; 07; 146).
In April 1990, cult members sprayed what they mistakenly believed was botulinum toxin from three trucks as they drove near two U.S. naval bases, Narita International Airport (one of two international airports serving the Greater Tokyo Area; also known as Tokyo-Narita, and formerly and originally known as New Tokyo International Airport), the National Diet (the National Legislature of Japan), the Imperial Palace, and the headquarters of a rival religious group (05). The goal was partly retaliation for the defeat of cult members who were running for political office and partly an attempt to precipitate an apocalyptic war.
On June 9, 1993, cult members sprayed what they mistakenly believed was botulinum toxin from a car equipped with a spraying device as it drove in Tokyo. The goal was to disrupt Prince Naruhito's wedding, seize power, and place the blame on the United States.
In the fall of 1994, cult members attempted to kill an attorney, Taro Takimoto, by mixing what they mistakenly believed was botulinum toxin into his drink. Takimoto had been assisting cult members who wished to leave the group and had been working on behalf of Aum victims.
On March 15, 1995, cult members attempted to spread what they mistakenly believed was botulinum toxin in the Kasumigaseki, Tokyo, subway station by placing briefcases equipped with custom spraying devices near the ticket barriers. The presumptive toxin had been replaced with a nontoxic substance (possibly water) by a dissident cult member. In any case, as in the cult's prior efforts, the cult had failed to acquire the necessary strain of Clostridium botulinum from which the botulinum toxin is derived.
Impact of infectious disease disinformation on national security. Perhaps the most significant underrecognized problem associated with COVID-19 is how disinformation weakened confidence in public health infrastructure and governments, something malicious adversaries have demonstrated that they can exploit (148; 141; 87). "Disinfodemic" — a portmanteau between “disinformation” and “epidemic” —refers to the wide and rapid accumulation and dissemination of disinformation about a given subject. As facts, rumors, and fears mix and disperse, a disinfodemic creates loud background noise, preventing the general public from discerning between accurate and false information (06). The COVID-19 disinfodemic helped erode public trust in public health, scientists, and governments and created barriers to controlling COVID-19 (06).
Government-sponsored disinformation campaigns have been positively associated with infectious disease epidemics, including COVID-19, over the last two decades. After controlling for climatic, public health, socioeconomic, and political factors, government-sponsored disinformation was significantly associated with the incidence and prevalence percentages of respiratory infections in susceptible populations from 2001 to 2019 (97); in particular, disinformation was significantly associated with the incidence rate ratio of cases of COVID-19.
Anti-vaccine messages primarily (1) focused on values related to safety, political or conspiracy theories, and choice, and (2) used approaches to instigate fear over the perceived severity and perceived susceptibility of adverse outcomes related to vaccines (132), all of which can be manipulated using bots on social media. Disinformation campaigns targeting health crisis communication during the COVID-19 pandemic sought to cripple the medical response to the novel virus and use the pandemic for political purposes (113). Propaganda from Russia sought to infiltrate public and social media in Ukraine (113). The United Kingdom and the European Union passed legislation criminalizing the malicious coronavirus falsehoods, including in relation to public health measures (122).
Disinformation on COVID-19 was amplified by coaching social media users to mass share the disinformation, by promoting activism to maximize the speed at which the disinformation was propagated, and by promoting negative sentiments against public health interventions such as vaccination and containment measures (109; 03). This was particularly effective because the campaign established a decentralized information-sharing network, effectively subverting efforts to gatekeep its misinformation (109).
There are often real events behind mis- or disinformation trends, which unverified sources misrepresent or take out of context (89). The most common hoaxes on science and health during the COVID-19 pandemic involved information on scientific research or health management, used text, were based on deception, used real sources, were international in scope, and were spread through social networks (96).
Mis- and disinformation about the pandemic compounded and exacerbated the security challenges posed by a serious public health crisis (03; 12). Disinformation often relied on existing narratives within right-wing populism movements to increase mistrust and discontent (12). These largely right-wing populist narratives broadened the gap between states and people, weakened public compliance with state health security measures, and increased fragmentation, polarization, and discrimination, impacting societal trust (12).
Involvement of the nervous system is uncommon with most microorganisms that might reasonably be employed in biological warfare and bioterrorism.
Bacillus anthracis. Severe hemorrhagic meningitis regularly occurs with anthrax infection, often accompanied by severe systemic disease, most likely transmitted by spore inhalation.
All forms of anthrax infection (ie, cutaneous, gastrointestinal, inhalational, and injection) can be complicated by meningitis. With natural anthrax, anthrax bacilli most commonly enter the body via the skin and disseminate to the CNS via the hematogenous or lymphatic routes, leading to fatal bacterial meningitis. The interval from exposure to presentation of cardinal signs (ie, fever, dyspnea, and headache) varies from 2 to 60 days.
Botulinum toxin. At the end of the 18th century, outbreaks of "sausage poisoning" in southern Germany, especially in Württemberg, prompted early research on botulism. The German poet and district medical officer Justinus Kerner (1786-1862) published the first accurate descriptions of the symptoms of food-borne botulism between 1817 and 1822 (63). Kerner labeled the suspected poison "sausage poison" or "fatty poison."
Clostridium botulinum was first recognized and isolated in 1895 by Belgian bacteriologist Émile Pierre-Marie van Ermengem (1851-1932), a professor of bacteriology at the University of Ghent. van Ermengem investigated a botulism outbreak in the small Belgian village of Ellezelles that occurred after a funeral dinner with home-cured ham in which 34 people were poisoned (156). The isolate was originally named Bacillus botulinus, after the Latin word for sausage, botulus. However, isolates from subsequent outbreaks were always found to be anaerobic spore formers, so American bacteriologist Ida Albertina Bengtson (1881-1952) proposed that the organism be placed in the genus Clostridium as the genus Bacillus was restricted to aerobic spore-forming rods (11). Bengtson had earned her master's degree in 1913 and her PhD in 1919, both from the University of Chicago. In 1916, she became the first woman hired to work in the Hygienic Laboratory of the U.S. Public Health Service (later part of the National Institutes of Health).
At her microscope, this historic image depicts bacteriologist Dr. Ida A. Bengtson (1881-1952), while at work in her laboratory. The photograph was taken from the U.S. Public Health Service records. Dr. Bengtson was particularly...
Natural outbreaks of foodborne botulism are typically from ingestion of improperly prepared home-canned foods (41; 43; 140; 157; 93; 70), although botulism from drinking illicit alcohol has been reported (102). Botulism may also occur with the injection of illicit drugs, such as black tar heroin or methamphetamine (116; 159), and contamination of wounds. In foodborne outbreaks in North America in which the toxin type was determined, most were caused by type A, occasionally by type B or type E, and rarely by type F (140); however, in outbreaks among Alaska Native communities, particularly the Inuit of Nunavik in northern Quebec and the First Nations population of the Pacific coast of British Columbia, those affected are predominantly exposed to type E botulinum toxin through the consumption of traditionally prepared marine mammal and fish products (43; 93; 70). Other countries have somewhat different distributions of toxin types; in France, for example, type B has been the predominant toxin type, although a wider diversity of toxin types has been noted since 2000 (126).
Jars containing contaminated Jalapeño peppers preserves, which were involved in an outbreak of botulism in Pontiac, Michigan in April 1977. (Source: Photograph by Dr. Chas Hatheway, Centers for Disease Control and Prevention, ...
Data are derived from routine, passive national surveillance. Data are presented as one trend line because the incidence and absolute case count trend lines are indistinguishable. (Source: Varma JK, Katsitadze G, Moiscrafishvil...
Botulism cases (n = 31) in a federal correctional facility, by reported date of hooch exposure and symptom onset. Hooch is defined as an illicitly made alcoholic beverage. First exposure is defined as the first exposure to hooc...
(Source: Centers for Disease Control and Prevention; Peak CM, Rosen H, Kamali A, et al. Wound botulism outbreak among persons who use black tar heroin - San Diego County, California, 2017-2018. MMWR Morb Mortal Wkly Rep 2019;67...
Cases by epidemiologic week of symptom onset. (Source: Peak CM, Rosen H, Kamali A, et al. Wound botulism outbreak among persons who use black tar heroin - San Diego County, California, 2017-2018. MMWR Morb Mortal Wkly Rep 2019;...
(Source: Waltenburg MA, Larson VA, Naor EH, et al. Notes from the field: botulism type B After intravenous methamphetamine use--New Jersey, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1425-6. Public domain.)
(A) Length of hospitalization, (B) length of time in a special care unit. The box and whiskers represent the data as quartiles; the whiskers (vertical lines) represent the top and bottom values, the box represents the 1st (bott...
Bioterrorist attacks with botulinum toxin could be either through oral ingestion or inhalation (59). Previous natural cases of inhalation botulism are rare (74; 130; 126; 131). The characteristic descending paralysis in humans typically starts in the extraocular and bulbar muscles, with associated autonomic features. Bilateral but asymmetric clinical signs, although rare in botulism, do not rule out that diagnosis; in particular, asymmetry may occur with demyelination of cranial nerves (64). Repetitive nerve stimulation usually shows an incremental muscle response. The differential diagnosis is from naturally occurring paralyzing illnesses (eg, Guillain-Barré syndrome, myasthenic crisis, diphtheria, tetrodotoxin, saxitoxin, snake envenomation) and from chemical warfare poisoning by organophosphates. Treatment is supportive.
(Source: Photograph by Dr. VR Dowell Jr., Centers for Disease Control and Prevention, 1980. Public Health Image Library ID# 16681, Centers for Disease Control and Prevention, Atlanta, Georgia. Public domain.)
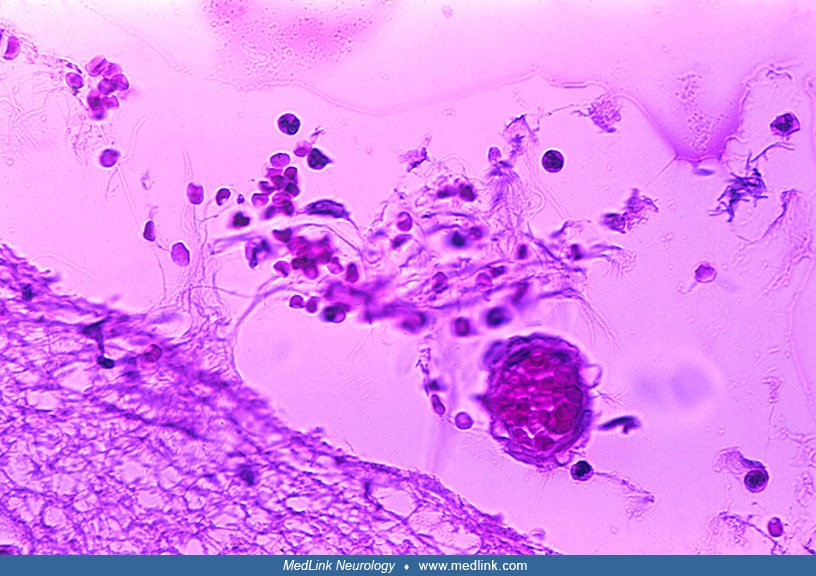
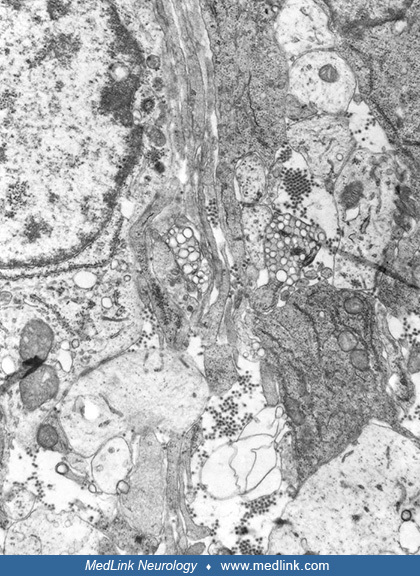
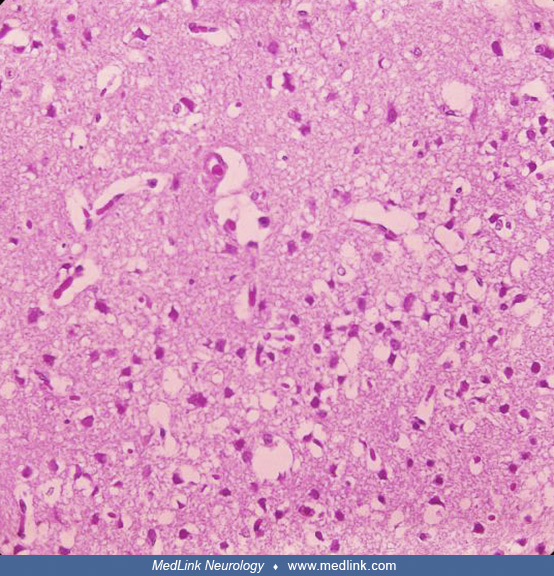
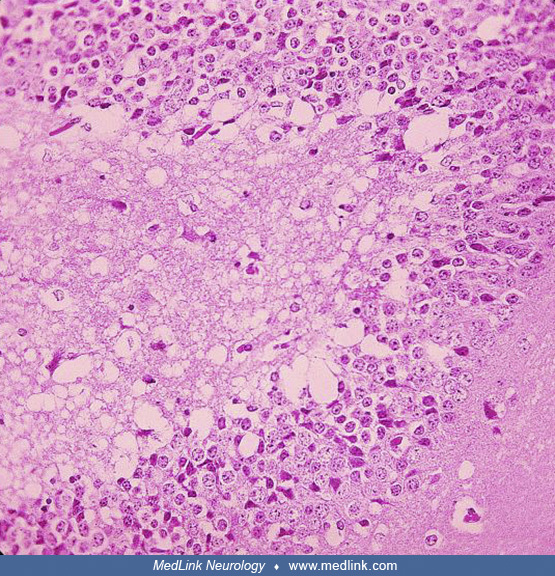
Postmortem cranial nerve tissue from a patient with asymmetric Type F botulism showing fragmentation of myelin sheaths and inflammatory infiltration of B and CD3+ T-cells within the nerve tissue (original magnification ×200). (...
Postmortem cranial nerve tissue from a patient with asymmetric Type F botulism showing B-cell infiltration of nerve tissue. (Source: Filozov A, Kattan JA, Jitendranath L, et al. Asymmetric type F botulism with cranial nerve dem...
Postmortem cranial nerve tissue from a patient with asymmetric Type F botulism showing CD68-positive myelinoklastic macrophages (original magnification ×400). (Source: Filozov A, Kattan JA, Jitendranath L, et al. Asymmetric typ...
Bioterrorists are more likely to contaminate victims’ inhaled air with botulinum toxin rather than the food supply. No immediate effects are noted after inhalation of botulinum toxin as the latent period varies from 1 to 5 days. Depending on the dissemination method, such attacks would likely affect both animals and humans.
The clinical features in humans are predominantly a combination of multiple cranial nerve palsies and skeletal muscular paralysis. The early manifestations of botulin intoxication are usually ophthalmological, with symptoms such as blurred vision, diplopia, and ptosis. Signs include nystagmus, mydriasis, and extraocular muscle dysfunction. Other symptoms, such as dysphagia and dysarthria, are associated with descending flaccid paralysis that typically develops 12 to 72 hours after exposure. The autonomic effects of botulism are manifested by anticholinergic signs and symptoms (eg, dry mouth, ileus, constipation, and urinary retention). Sensory symptoms are absent. Because botulinum toxins do not cross the blood-brain barrier and do not affect the brain, affected individuals are usually alert and oriented. With severe respiratory muscle paralysis, the patient may become cyanotic or exhibit narcosis from CO2 retention. Respiratory paralysis may lead to death.
Anthrax. Untreated, the case fatality from cutaneous anthrax is about 20% to 30%, compared to 25% to 60% with gastrointestinal anthrax, and nearly 100% with inhalational anthrax. With treatment, mortality is less than 1% with cutaneous anthrax (47; 57; 166; 71), whereas mortality remains high, even with aggressive treatment, with gastrointestinal or inhalational anthrax (73; 71). Inhalational anthrax was generally thought to be fatal in 80% to over 90% of cases (119; 18; 01; 166), but the mortality in the 2001 U.S. outbreak has been much better than anticipated (5 of 11 cases or 45%) with the use of aggressive treatment and intensive care unit support, including mechanical ventilation and dialysis as necessary (27; 166). From 2001 to 2014, 8 of 15 (53%) patients with inhalation anthrax survived with early diagnosis, combination antimicrobial drug treatment to eradicate the bacteria and inhibit toxin production, and aggressive pleural effusion management (71). Initiation of antibiotic or anthrax antiserum therapy during the prodromal phase of inhalational anthrax is associated with markedly improved survival compared with initiation of treatment of fulminant cases (73). Nevertheless, anthrax survivors report significant health problems and poor life adjustment 1 year after the onset of bioterrorism-related anthrax infection (129).
Anthrax meningoencephalitis is usually rapidly fatal, with roughly two-thirds of affected patients dying within 24 hours of presentation (92; 112), although there are a small number of reported survivals following anthrax meningoencephalitis (51; 135; 160; 149; 151; 85; 147; 92; 13). In a systematic review, survival of anthrax meningitis was predicted by treatment with a bactericidal agent and the use of multiple antimicrobials (82). There is limited information on long-term outcomes among the few survivors, but several cases were reported to have fully recovered (149; 147; 92; 13).
Botulism. The advent of modern supportive care has improved botulism prognosis so that the case fatality rate for food-borne botulism has decreased from the 50% to 70% range to the 5% to 20% range. Although botulinum neurotoxins bind irreversibly to presynaptic endplates and impair them irreparably, axons can regenerate new endplates, and full recovery can occur, although it may take several months. The case fatality and outcomes from biological warfare or terrorism could easily be much worse than the results obtained with individual sporadic cases or cases from small natural outbreaks because the medical capacity could be overwhelmed and treatment supplies (eg, Botulism Antitoxin Heptavalent [A, B, C, D, E, F, G]) could be exhausted by sudden widescale demand.
Various agents used for biological and chemical warfare are listed in Table 1. Many of them can cause neurologic manifestations. Encephalomyelitic viruses, toxins, and chemicals are more likely to be associated with neurotoxicity. Some of the bacterial infections may be associated with neurologic complications.
Characteristics that make a pathogen a high risk for bioterrorism include a low infective dose, ability to be aerosolized, high contagiousness, and hardiness (ie, survival in various environmental conditions) (04).
|
Bacteria |
• Bacillus anthracis (inhalation anthrax) |
|
Viruses |
• Eastern equine encephalitis |
|
Toxins |
• Botulinum |
CDC categorization of bioterrorism agents and diseases. The Centers for Disease Control and Prevention (CDC) has a 3-tier categorization system for bioterrorism agents and diseases (CDC: Bioterrorism Agents and Diseases):
Category A. Category A is comprised of high-priority agents that pose a risk to national security because (1) they can be easily disseminated or transmitted from person to person, (2) they have the potential for major public health impact (eg, high case-fatality rates), (3) they will likely cause public panic and social disruption, and (4) they require special measures for public health preparedness. The two Category A diseases with the greatest potential to affect the nervous system as a major feature of the illness are anthrax (Bacillus anthracis) and botulism (Clostridium botulinum toxin). Other Category A diseases or agents include plague (Yersinia pestis), smallpox (variola major), tularemia (Francisella tularensis), and viral hemorrhagic fevers (eg, Ebola, Marburg) (08; 09; 136).
Category B. Category B is comprised of moderate-priority agents that (1) are moderately easy to disseminate, (2) result in moderate morbidity rates and low mortality rates, and (3) require specific enhancements for diagnostic capacity and warrant enhanced disease surveillance. Of these, only viral encephalitis viruses (alphaviruses, such as eastern equine encephalitis, Venezuelan equine encephalitis, and western equine encephalitis) are likely to primarily affect the nervous system (137).
Noncontrast CT scan of the brain of a 5-year-old girl with eastern equine encephalitis on hospital day 2 showing subtle hypoattenuation of the left caudate head (arrow) and diencephalic region. (Source: Silverman MA, Misasi J, ...
Axial FLAIR image from a brain MRI scan of a 13-year-old boy with eastern equine encephalitis on hospital day 2 showing abnormal T2 hyperintense regions of the bimesial temporal regions (thick arrows) with accompanying abnormal...
Axial FLAIR from a brain MRI scan of a 3.5-year-old girl with eastern equine encephalitis on hospital day 3 showing abnormal T2 hyperintense caudate and thalamic nuclei, most prominent on the right (arrow). (Source: Silverman M...
Axial FLAIR from a brain MRI scan of a 3.5-year-old girl with eastern equine encephalitis on hospital day 3. The abnormal T2 hyperintense regions are most prominent in the right parietotemporal gray matter (arrow) and subcortic...
Histopathologic features from a 5-year-old girl with eastern equine encephalitis in 2005, as part of a study of eastern equine encephalitis in Massachusetts and New Hampshire from 1970 to 2010. The postmortem samples of central...
Histopathologic features from a 5-year-old girl with eastern equine encephalitis in 2005, as part of a study of eastern equine encephalitis in Massachusetts and New Hampshire from 1970 to 2010. The postmortem samples of central...
Histopathologic features from a 5-year-old girl with eastern equine encephalitis in 2005, as part of a study of eastern equine encephalitis in Massachusetts and New Hampshire from 1970 to 2010. The postmortem samples of central...
Histopathologic features from a 5-year-old girl with eastern equine encephalitis in 2005, as part of a study of eastern equine encephalitis in Massachusetts and New Hampshire from 1970 to 2010. The postmortem samples of central...
Category C. Category C is comprised of lower-priority agents, including emerging pathogens that could potentially be engineered for mass dissemination because of availability, ease of production and dissemination, or potential for major health impact (eg, potential for high morbidity or mortality).
Bacillus anthracis (Category A). Insight regarding the mechanism for the spread of B anthracis by inhalation is provided by pathologic studies of victims from the Sverdlovsk epidemic of 1979. The contagion mechanism was traced to the release of aerosols containing this organism at a secret biological-agent production facility in Russia (68).
A hematogenous spread involved the central nervous system, resulting in vasculitis, hemorrhages, and edema due to the toxins. The risk of hemorrhagic meningitis after exposure to anthrax inhalation is estimated to be as high as 50% (76; 77).
Equine encephalitis (Category B). Venezuelan equine encephalitis virus could be developed for transmission by inhalation with secondary zoonotic transmission cycles sustained by horses and mosquitoes (59), as could eastern and western equine encephalitis viruses.
The Eastern equine encephalitis virus is spread to people by the bite of an infected mosquito (19).
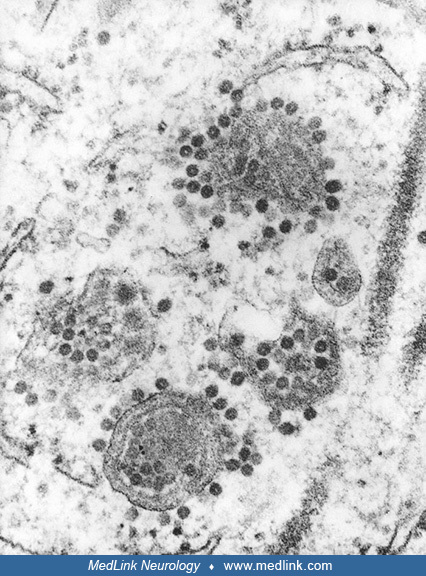
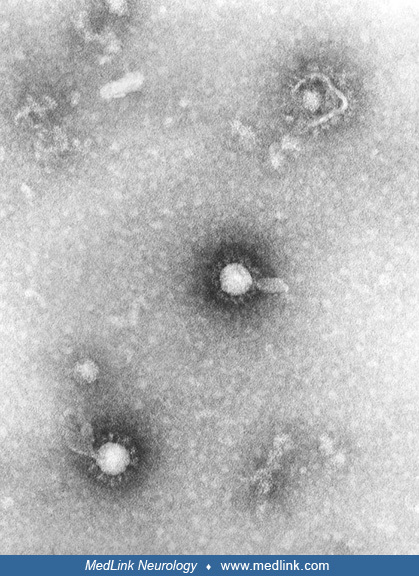
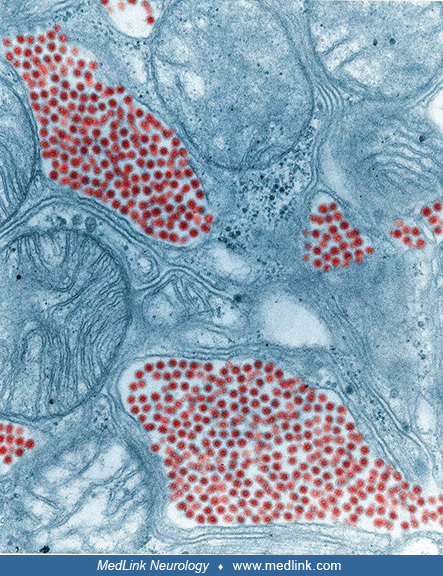
Transmission electron microscopic image reveals numerous Eastern equine encephalitis virus particles harbored in this mosquito salivary gland tissue specimen. Photomicrograph by CDC/Dr. Fred Murphy and Sylvia Whitfield in 1975....
Transmission electron microscopic image reveals numerous Eastern equine encephalitis virus virions in this central nervous system tissue specimen. Photomicrograph by CDC/Dr. Fred Murphy and Sylvia Whitfield in 1975. (Source: Pu...
Transmission electron microscopic image reveals numerous Eastern equine encephalitis virus virions in this specimen of central nervous system tissue. Photomicrograph by CDC/Dr. Fred Murphy and Sylvia Whitfield in 1975. (Source:...
Transmission electron microscopic image reveals numerous Eastern equine encephalitis virus virions in this specimen of central nervous system tissue. Photomicrograph by CDC/Dr. Fred Murphy and Sylvia Whitfield in 1975. (Source:...
In this digitally colorized transmission electron microscopic image, the viral particles have been colorized red (magnification x 83,900) Photomicrograph by CDC/Dr. Fred Murphy and Sylvia Whitfield in 1975. (Source: Public Heal...
In this transmission electron microscopic image, note the numerous Eastern equine encephalitis virions in various stages of development, from the precursor particles through the mature virus. Image by CDC, 1969. (Source: Public...
In the United States, most cases occur in eastern or Gulf Coast states.
Although rare, approximately 30% of people with Eastern equine encephalitis die, and many survivors have residual neurologic problems. Eastern equine encephalitis, commonly called Triple E or sleeping sickness (not to be confused with African trypanosomiasis), is caused by a zoonotic mosquito-vectored Togavirus that is present in North, Central, and South America and the Caribbean.
In the United States, Eastern equine encephalitis is often associated with coastal plains, most commonly in East Coast and Gulf Coast states. No human vaccines or licensed therapeutic drugs are available for Eastern equine encephalitis in the U.S. Eastern equine encephalitis virus is a potential bioweapon, particularly because it is transmissible by aerosol.
The Western equine encephalomyelitis virus is an alphavirus of the family Togaviridae.
The Western equine encephalomyelitis virus is an arbovirus (arthropod-borne virus) transmitted by mosquitoes of the genera Culex and Culiseta.
The virus is transmitted to people and horses by bites from infected mosquitoes (Culex tarsalis and Aedes taeniorhynchus), particularly during wet summer months; birds are the natural reservoir of the virus.
In North America, Western equine encephalomyelitis occurs primarily in U.S. states and Canadian provinces west of the Mississippi River. The disease also occurs in South America. Western equine encephalomyelitis is commonly a subclinical infection in adult humans, but the disease can cause serious sequelae in infants, children, and the elderly. The overall mortality of Western equine encephalomyelitis is low (approximately 4%) and is associated mostly with infection in the elderly. Approximately 15% to 20% of horses that acquire the virus die or need to be euthanized.
No human vaccines or licensed therapeutic drugs are available in the U.S. for Western equine encephalomyelitis. Western equine encephalitis virus was one of more than a dozen agents the United States researched as potential biological weapons before the nation suspended its biological weapons program.
Venezuelan equine encephalitis is a mosquito-borne disease endemic in regions of Central and South America that causes sporadic outbreaks of equine and human encephalitis. The virus induces neural necrosis and edema in affected mammals.
As in humans, under natural conditions (as opposed to the artificial condition of bioweapons use), the etiologic pathogens responsible for equine encephalitic diseases are transmitted to horses by mosquito vectors, with various...
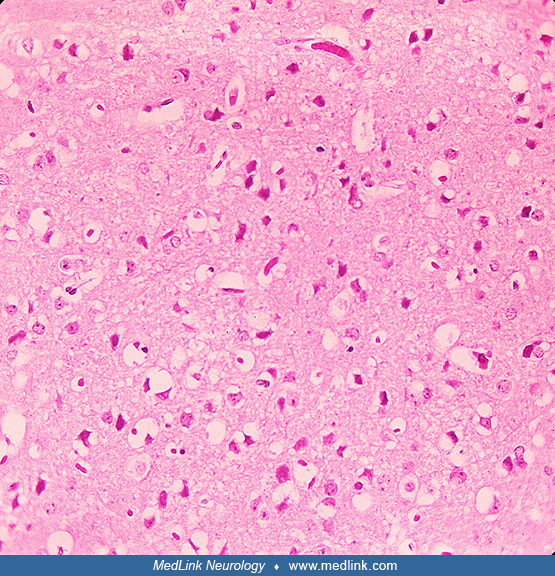
This photomicrograph of a mouse brain tissue sample collected after the mouse had succumbed to Venezuelan equine encephalitis reveals histopathologic changes indicative of neural necrosis and edema. Photomicrograph by CDC/Dr. F...
This photomicrograph of a mouse brain tissue sample collected after the mouse had succumbed to Venezuelan equine encephalitis reveals histopathologic changes indicative of neural necrosis and edema (magnification x 300). Photom...
This photomicrograph of a mouse brain tissue sample collected after the mouse had succumbed to Venezuelan equine encephalitis reveals histopathologic changes indicative of neural necrosis and edema (magnification x 300). Photom...
This photomicrograph of a mouse brain tissue sample collected after the mouse had succumbed to Venezuelan equine encephalitis reveals histopathologic changes indicative of neural necrosis and edema (magnification x 300.) Photom...
This photomicrograph of a mouse brain tissue sample collected after the mouse had succumbed to Venezuelan equine encephalitis reveals histopathologic changes indicative of neural necrosis and edema (magnification x 300). Photom...
This negatively stained, transmission electron microscopic image revealed Venezuelan equine encephalitis virus virions in the tissue specimen, which had been fixed using phosphotungstic acid. Phosphotungstic acid is very electr...
Toxins. The term “toxin” refers to a toxic substance of biological origin, although simple toxins can be synthesized in the laboratory or produced by genetic modification in other species.
Bacterial toxins are of two general types: proteinaceous exotoxins (eg, tetanus, diphtheria, or botulinum toxins) are part of a defensive system of bacteria (to avoid capture, ingestion, etc.) and so are typically secreted into the surrounding medium, in contrast to endotoxins (eg, lipopolysaccharides in the outer membrane of gram-negative bacteria) that are only released when the bacterium disintegrates.
Mycotoxins are diverse and could be used by terrorist organizations to poison food and water sources, although dispersal in indoor air appears to be the most effective method for a bioterrorist attack (99). Crude concentrated or dried extracts of readily grown fungal cultures can simultaneously produce several toxins with synergistic actions, but mycotoxin bioterror weapons are more likely to involve the liver (eg, aflatoxins, fumonisin B1), kidneys (ochratoxin A, fumonisin B1), lungs (fumonisin B1), gastrointestinal tract (trichothecenes type A, eg, T-2 toxin; type B, eg, deoxynivalenol [DON]), and bone marrow (trichothecenes type A, eg, T-2 toxin) than the nervous system (98; 72; 142; 114; 99; 80).
As chemical products of living organisms, toxins, when employed by people for a nefarious purpose, occupy an ill-defined “no man’s land” between chemical warfare and biological warfare agents. From a pharmacological and toxicological point of view, toxins could be considered chemical weapons, but most experts and the U.S. Army classify toxins as biological weapons.
Toxins most relevant to bioterrorism include ricin, botulinum, Clostridium perfringens epsilon toxin, conotoxins, Shiga toxins, saxitoxins, tetrodotoxins, mycotoxins, nicotine (04), and brevetoxin. Of these, botulinum, brevetoxin, conotoxins, nicotine, saxitoxins, and tetrodotoxin are most likely to involve the nervous system.
(1) Botulinum toxin (Category A). Botulinum toxin is derived from anaerobic Clostridium botulinum bacteria. Clostridium botulinum bacteria are Gram-positive. On microscopic examination after appropriate histologic staining, Clostridium botulinum endospores may have the morphology of “safety pins,” ”bobby pins,” or “tennis rackets.”
Close view of the surface of a blood agar culture plate, which produced these colonies after having been inoculated with Clostridium botulinum, type-A, strain 2 bacteria and incubated for 48 hours. (Source: Photograph by Dr. Ho...
It is feasible to deliver botulinum toxin as an aerosolized biological weapon. Botulinum toxins are a series of seven related protein neurotoxins, serotypes A through G.
(Source: Wikimedia Commons. Created on October 4, 2008. See also: Lacy DB, Tepp W, Cohen AC, DasGupta BR, Stevens RC. Crystal structure of botulinum neurotoxin type A and implications for toxicity. Nat Struct Biol 1998;5[10]:89...
Each serotype is a protein of approximately 150 kD produced by a separate strain of the gram-positive anaerobic bacterium Clostridium botulinum. Botulinum toxins block acetylcholine release from the presynaptic nerve terminal in the peripheral nervous system, leading to muscle paralysis. Botulinum toxin A is the most toxic serotype for humans. It can be readily aerosolized and persists in this state for weeks in still water and food but degrades on exposure to heat, acidity, or air for more than 12 hours.
Botulinum toxin binds to high-affinity recognition sites on the cholinergic nerve terminals (there may be a separate one for each serotype). The toxin enters the nerve terminal by receptor-mediated endocytosis but then is extruded into the cytoplasm. In the cytoplasm, the toxin cleaves SNARE proteins (ie, proteins that mediate vesicle fusion with the presynaptic membrane), preventing the cell from releasing vesicles of the neurotransmitter, interrupting neurotransmission, and causing paralysis (61; 44).
Botulinum toxin consists of two polypeptide subunits: A and B chains. The B subunit binds to receptors on the axons of motor neurons. The toxin is taken into the axon by receptor-mediated endocytosis, and then the A chain acts to prevent fusion of the synaptic vesicle with the cell membrane, thus preventing the release of acetylcholine. This mechanism of blocking neuromuscular transmission is called presynaptic inhibition. The presynaptic inhibition interrupts both cholinergic autonomic (muscarinic) and motor (nicotinic) neurotransmission, producing the cranial neuropathies and skeletal muscle paralysis seen in clinical botulism. Recovery only occurs after the neuron generates a new axon terminal, which can take months.
The estimated lethal dose of type A toxin is 1.3 to 2.1 ng/kg intravenously or intramuscularly, 10 to 13 ng/kg when inhaled, or 1000 ng/kg orally.
(2) Brevetoxins (Category C). Brevetoxins are a group of tasteless and odorless neurotoxic cyclic polyether compounds produced naturally by species of dinoflagellates (Karenia sp.). Brevetoxins bind to voltage-gated sodium channels in nerve cells, leading to disruption of normal neurologic processes and causing neurotoxic shellfish poisoning. The typical natural route of human exposure is by ingestion of contaminated shellfish. Ingestion of these toxins produces a combination of gastrointestinal and neurologic signs and symptoms; gastrointestinal symptoms include abdominal pain, vomiting, and diarrhea, and the most disabling neurologic signs and symptoms include vertigo and ataxia. Inhalational exposure may cause respiratory symptoms, such as cough, dyspnea, and bronchospasm. Toxicity can also result from dermal exposure.
(3) Conotoxins (Category C). Conotoxins, the venom of various species of cone snails, are pharmacologically and chemically diverse.
Conotoxins affect neurotransmitter receptors (eg, nicotinic, adrenergic, NMDA, and serotonergic) and ion channels (eg, sodium, potassium, and calcium).
Shown is Arg13 in μ-conotoxin GIIIA, which is critical for the channel block. (Source: Tikhonov DB, Zhorov BS. Predicting structural details of the sodium channel pore basing on animal toxin studies. Front Pharmacol 2018;9:880....
A μ-conotoxin (μ-CTX) mutant does not completely block the sodium current. The sodium ion can escape through the incompletely sealed outer pore and vacate the cation-attractive central cavity for binding of QX-222, which is a l...
The most lethal effect of conotoxins on humans is diaphragmatic paralysis and respiratory arrest. Most conotoxins are not a bioterrorism threat, but some could be weaponized and used as an aerosol (eg, a-conotoxins, k-conotoxins, and o-conotoxins).
(4) Saxitoxins (Category C). More than 50 structurally related neurotoxins (known collectively as "saxitoxins") are produced by protists, algae, and cyanobacteria. Saxitoxin, the best-known paralytic shellfish toxin, is one of the most potent known natural toxins. Saxitoxin acts as a selective, reversible, voltage-gated sodium channel blocker. Saxitoxin binds directly in the pore of the channel protein, occluding the opening, and preventing the flow of sodium ions through the membrane.
(5) Tetrodotoxin (Category C). Tetrodotoxin is an extremely potent toxin naturally found mainly in the liver and sex organs (gonads) of some fish (eg, puffer fish, globefish, and toadfish) and in some amphibian, octopus, and shellfish species.
Tetrodotoxin binds to the voltage-gated sodium channels in nerve cell membranes and blocks the passage of sodium ions (responsible for the rising phase of an action potential) into the neuron (94). This mechanism of action was established in 1964 by Toshio Narahashi (1927-2013), John W Moore (1920-2019), and William R Scott at Duke University (108; 107).
Repeats I, II, III, and IV are green, yellow, cyan, and magenta, respectively. The outer carboxylates are shown as sticks. (Source: Tikhonov DB, Zhorov BS. Predicting structural details of the sodium channel pore basing on anim...
In the schematic, the permanently charged QX-222 reaches the inner pore via III/IV repeat interface and displaces the resident ion that leaves the central cavity through the selectivity filter. QX-222, a lidocaine derivative, i...
When the outer pore is blocked by tetrodotoxin (TTX), the sodium ion is trapped in the central cavity and prevents binding of QX-222. QX-222, a lidocaine derivative, is a sodium channel blocker. (Source: Tikhonov DB, Zhorov BS....
Brevetoxins, saxitoxins, tetrodotoxin, and some conotoxins all act to block voltage-gated sodium channels.
Positions of the selectivity filter "DEKA residues" and the outer carboxylates, which interact with TTX (tetrodotoxin), STX (saxitoxin), and μ-CTX (μ-conotoxin), are marked by red dots. (Source: Tikhonov DB, Zhorov BS. Predicti...
Positions of toxin-interacting residues are shown by red circles. In eukaryotic voltage-gated sodium channels, the selectivity filter DEKA ring, which contains D, E, K, and A residues, borders the extracellularly exposed outer ...
The most likely first indicator of a biological warfare event would be a marked increase in the number of patients presenting with clinical features caused by the disease agent, beyond what would be expected for natural outbreaks. Patients presenting en masse with a similar pulmonary syndrome should alert the clinician to the possibility of aerosolized toxin exposure (167).
If an attack with a toxin were to occur, epidemiological clues would include the clustering of cases (ie, more than expected with the natural occurrence of the disease), more severe disease than typical, and unusual routes of exposure (eg, inhalation rather than ingestion for some toxins).
Anthrax bioweapon or bioterrorism. Anthrax is rarely found in most developed countries because of vaccines for animals and at-risk people. There has been a dramatic reduction in livestock and human anthrax cases in the United States since 1900 because of livestock and at-risk human immunization, proper disposal of infected animals, and restriction of imported wool, hides, and other products (17; 18).
Because of the complexities of the development and dispersal of an anthrax bioweapon, it had been anticipated that small-scale sabotage or attempts at personal murder were more likely than large-scale attempts at inflicting mass casualties with anthrax and that crude dispersal in a close area was the most likely mode of attack. In the 2001 United States outbreak of anthrax acquired through intentional contamination of mail, most cases involved media, government, and postal service employees; several civilian cases remain unexplained (28).
Epidemiologic clues to bioweapon use include (1) an outbreak or multiple, simultaneous outbreaks with large numbers of patients complaining of respiratory symptoms; (2) high case fatality numbers; (3) sick or dead animals of different species; and (4) multidrug-resistant pathogens (47). A World Health Organization report estimated that the release of 50 kg of anthrax spores upwind of a city of 500,000 population would result in 125,000 infections and nearly 95,000 deaths (165). A report by the United States Congressional Office of Technology Assessment estimated between 130,000 and 3 million deaths following the aerosolized release of 100 kg of anthrax spores upwind of Washington, D.C. (110).
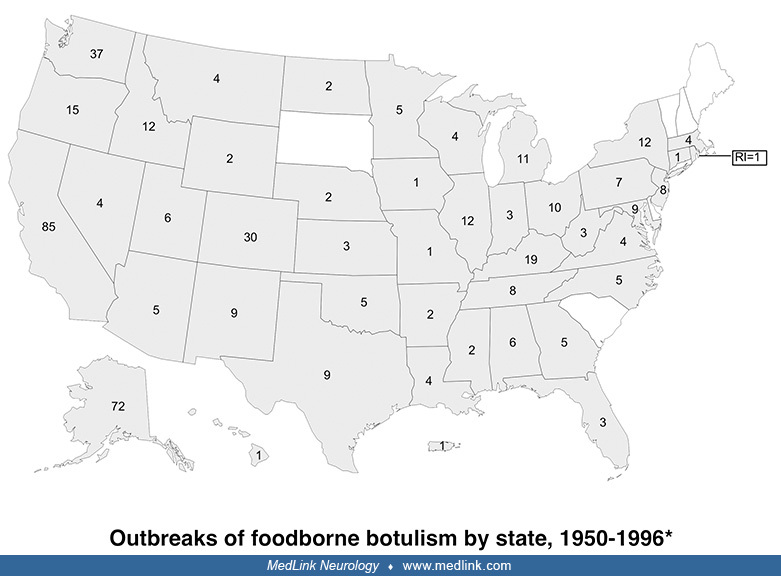
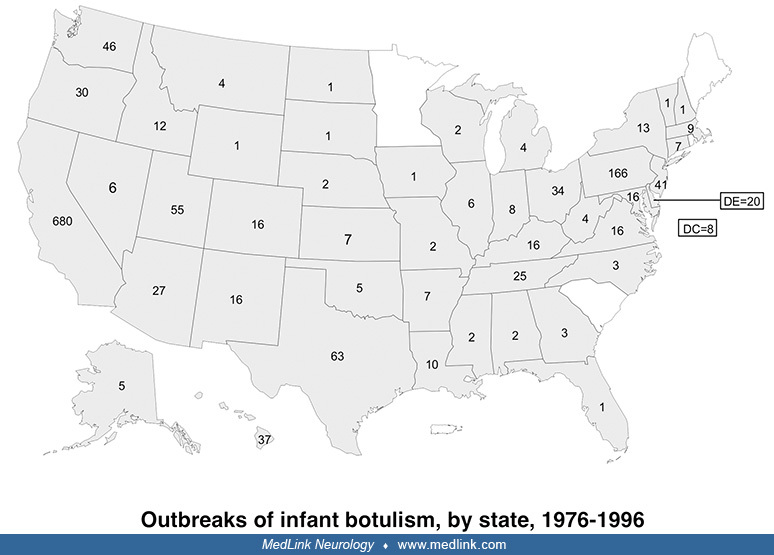
Botulism. An average of 110 cases of botulism are reported annually in the United States, about 25% of which are foodborne botulism, whereas 75% are infant botulism (25). Five western states account for more than half of reported foodborne outbreaks since 1950: California, Washington, Colorado, Oregon, and Alaska.
Foodborne botulism is a significant public health problem among Alaska Natives and is associated with improper preparation and storage of traditional foods. At least two thirds of foodborne outbreaks are due to home-processed foods, most of the remainder are from unknown sources, and only 7% are due to commercially processed foods (25).
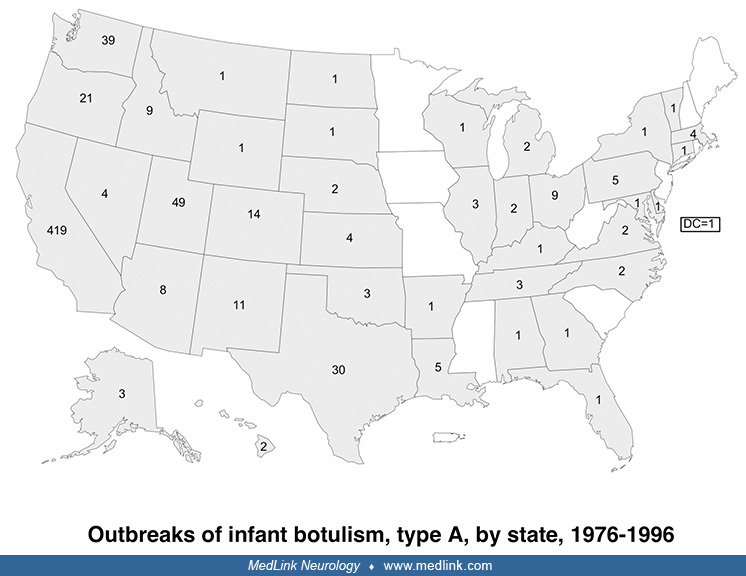
Although infant botulism has been the most common form of botulism in the U.S. since 1980, infant botulism is a rare sporadic disease that occurs in isolated cases, has no epidemic potential, and generally does not pose a major public health threat.
Infant botulism is epidemiologically distinct from foodborne botulism because it results from colonization (infection) of the intestine by spores of C. botulinum with subsequent in vivo toxin production rather than from ingestion of toxin preformed in contaminated foods. Although infant botulism was first described in 1976 (105; 117), earlier cases have been identified retrospectively (25).
Much work needs to be done internationally to develop capabilities to manage victims of biological or chemical terrorism (84). Most physicians and hospital emergency departments in the United States are ill-prepared to treat victims of such terrorism (83; 162; 143).
The United States military and the Department of Homeland Security regularly conduct bioterrorism response training, sometimes in conjunction with civilian HAZMAT firefighters and U.S. National Guard Weapons of Mass Destruction-Civil Support Teams (WMD-CST).
A Chemical, Biological, Radiological, and Nuclear (CBRN) Marine with CBRN platoon, Headquarters Battalion, 3rd Marine Division lies on a table as fellow Marines remove his gear after passing out in the field within a contaminat...
A Strike Team member with the New Jersey National Guard's 21st Weapons of Mass Destruction-Civil Support Team (WMD-CST), is scrubbed at a decontamination station by Picatinny Arsenal during a training exercise with the Picatinn...
A Strike Team member with the New Jersey National Guard's 21st Weapons of Mass Destruction-Civil Support Team (WMD-CST), prepares to get scrubbed at a decontamination station by Picatinny Arsenal during a training exercise with...
Some of the soldiers are Chemical, Biological, Radiological and Nuclear (CBRN) Marines. Scenarios included an aircraft leaking chemical agents, locating a chemical agent spill, and rescuing multiple casualties in the field or in a metro line (no doubt motivated by the deadly Tokyo subway sarin attack in 1995 conducted by the Aum Shinrikyo cult). Some of the training exercises are conducted in Northville, a fictional town at the Chemical, Ordnance, Biological, and Radiological (COBRA) Facility in Anniston, Alabama.
Technical Emergency Response Training students perform a final exercise in Northville, a fictional town at the Chemical, Ordnance, Biological, and Radiological facility. During the exercise, students work with and identify biol...
Technical Emergency Response Training students perform a final exercise in Northville, a fictional town at the Chemical, Ordnance, Biological, and Radiological facility. During the exercise, students work with and identify biol...
Technical Emergency Response Training students perform a final exercise in Northville, a fictional town at the Chemical, Ordnance, Biological, and Radiological facility. During the exercise, students work with and identify biol...
Anthrax. Prevention of anthrax infection in civilians due to bioterrorist activity is limited to prevention of exposure and postexposure prophylaxis (postexposure prophylaxis is considered under management). In military personnel and selected at-risk populations, prevention has also included vaccination (42); however, legal challenges resulted in a temporary halt of military vaccinations as of December 2003.
Defense Billboard #135. (Source: Department of Defense. American Forces Information Service. Defense Visual Information Center on January 1, 2000. Courtesy of the U.S. National Archives. National Archives Identifier: 6507549. P...
Although previous CDC guidance (26) indicated that an anthrax vaccine could be requested through the CDC, such a vaccine was not provided to civilians during the 2001 outbreak in the United States (28; 92). Subsequently, civilians deemed at high risk of exposure who had completed a 60-day course of antibiotics were offered postexposure anthrax vaccination (34; 78).
Anthrax vaccine adsorbed (AVA), a cell-free, noninfectious anthrax vaccine prepared from a noncapsulating, nonproteolytic anthrax strain, was licensed by the U.S. Food and Drug Administration in 1970 for soldiers, at-risk scientists and laboratory workers (ie, those working with anthrax), at-risk veterinarians and livestock handlers, and at-risk wool mill workers (155). AVA was reapproved for licensure by the Food and Drug Administration in 1985 and remains the only licensed human anthrax vaccine in the United States (166). AVA, now marketed as BioThrax® (Emergent BioSolutions, Lansing, Michigan), is licensed for use in nonpregnant adults aged 18 to 65 years who are at high risk of exposure.
AVA is a considerable improvement over previously available vaccines, including the uncharacterized whole-cell vaccines developed initially in 1881 (133). Originally it was administered as a series of six subcutaneous exposures over 18 months, followed by annual boosters for persons with continuing potential exposure risk (47; 155). In 2008, the CDC published the results of a four-arm randomized controlled trial comparing the original regimen administered by subcutaneous administration with the original regimen administered by intramuscular injection, a reduced dose schedule administered by intramuscular injection, and a placebo that received saline injections at the same intervals. Intramuscular injection significantly reduced the occurrence of injection-side reactions, and immunological priming of reduced-dose regimens proved noninferior to the original licensed regimen (101). For pre-event or pre-exposure prophylaxis, the United States Advisory Committee on Immunization Practices now recommends five 0.5-ml doses of AVA administered intramuscularly at 0 and 4 weeks and 6, 12, and 18 months, followed by annual 0.5-ml booster injections after the 18-month dose (166). A booster dose of AVA for preexposure prophylaxis can be given every 3 years instead of annually to persons not at high risk for exposure to Bacillus anthracis who have previously received the initial AVA three-dose priming and two-dose booster series and want to maintain protection (14; 14). People with contraindications to intramuscular injections (eg, coagulopathies) may continue to receive AVA by subcutaneous administration (166). In contrast to the revised vaccine administration schedule and route for pre-event or preexposure prophylaxis, the FDA decided in June 2009 to continue the preexisting postexposure prophylaxis protocol until new data become available; therefore, for postexposure prophylaxis, AVA should only be administered under an Investigative New Drug protocol or Emergency Use Authorization in a three-dose series (at 0, 2, and 4 weeks) by the subcutaneous route in conjunction with antimicrobial therapy for a minimum of 60 days (166).
The Department of Defense Anthrax Vaccine Immunization Program, announced in 1997 and instituted in 1998, administered 8.4 million doses to approximately 2.1 million military personnel from March 1998 through December 2008 (166). Although previously mandatory for military servicemen and women, a United States District Court ruled on October 27, 2004, that the Department of Defense can no longer inoculate troops without their consent. The vaccine is associated with frequent local reactions but only rare serious side effects sufficient to require hospitalization (approximately once per 200,000 doses) (155). In fact, in a study employing a historical cohort before the inception of the Department of Defense Anthrax Vaccine Immunization Program in 1998, hospitalization following anthrax vaccination was significantly less likely from any cause, diseases of the blood and blood-forming organs, and diseases of the respiratory system among 170,723 active-duty U.S. service members who were vaccinated with the anthrax vaccine (161).
The Vaccine Adverse Event Reporting System has monitored adverse events reported following vaccination with AVA. The most common adverse events that occurred after AVA administration (either alone or concurrently with other vaccines) were arthralgia (17%), headache (16%), pruritus (15%), pain (14%), injection-site erythema (13%), fever (11%), erythema (10%), pain at the injection site (10%), rash (10%), and myalgia (10%) (166). There is no convincing evidence of long-term adverse health effects associated with AVA administration (166). Vaccine postmarketing surveillance has identified no association between optic neuritis and receipt of the anthrax vaccine by members of the U.S. military (115).
AVA is neither recommended nor licensed for use during pregnancy, and the Department of Defense continues a policy of avoiding anthrax vaccination in pregnant women (42). AVA is now available under an investigational new drug application in combination with extended antibiotic treatment for civilians exposed to inhalational anthrax who have completed a 60-day course of antibiotics (34; 78).
Botulism. Vaccination with a pentavalent toxoid of Clostridium botulinum toxin types A, B, C, D, and E is available as an investigational new drug for preexposure prophylaxis. This product has been administered to several thousand volunteers and occupationally at-risk workers and induces serum antitoxin levels that correspond to protective levels in experimental animals. Adequate antibody levels are transiently induced after three injections but decline before the 1-year booster.
A history of exposure to neurotoxic biological warfare agents may narrow the differential diagnosis to the agents involved. In febrile patients with acute neurologic deterioration after suspected exposure to biological or chemical agents, anthrax should be considered in the differential diagnosis if there are the following findings: (1) dark necrotic pustules on the extremities, (2) Gram-positive rods in the CSF, and (3) multifocal areas of unexplained intracerebral hemorrhage on CT scans.
Botulism should be differentiated from other neurologic conditions, including Guillain-Barré syndrome, myasthenia gravis, diphtheria, tick paralysis, viral encephalitis, basilar artery stroke, toxic effects of drugs (eg, atropine and aminoglycoside antibiotics), and multiple cranial nerve palsies due to lesions involving the skull base. Once botulism is considered, it is important to differentiate the pulmonary inhalation form from the food-borne form. If bioterrorism is suspected, differentiation should be made between the chemical nerve agents, such as sarin and botulinum toxin exposure effects, as shown in Table 2 (124).
|
Botulinum toxin |
Sarin | |
|
Time from exposure to symptoms |
Hours |
Minutes |
|
Neurologic manifestations |
Progressive flaccid skeletal muscle paralysis and multiple cranial nerve palsies |
Convulsions |
|
Respiratory |
Normal respiration initially, but progresses to paralysis of respiratory muscles |
Dyspnea due to bronchoconstriction and bronchorrhea |
|
Ophthalmological findings |
Blurred vision, droopy eyelids, and large pupils |
Blurred vision and small pupils |
|
Sialorrhea |
No |
Yes |
|
Death |
In 2 to 3 days |
In minutes with high-level exposure |
|
Response to treatment with atropine |
None. May aggravate the condition. |
Yes |
Neurologic workup. Workup includes lumbar puncture with examination of CSF and brain imaging (CT or MRI). Normal CSF helps to differentiate botulism from other neurologic disorders, such as Guillain-Barré syndrome. Electrophysiological studies can provide presumptive proof of botulism in patients with the clinical signs of botulism; this is especially helpful when bioassay studies are negative. The most consistent electrophysiological abnormality is a small evoked muscle action potential in response to a single supramaximal nerve stimulus in a clinically affected muscle done pre- and postexercise (164).
If a biological attack occurs, clinical diagnosis should be supplemented by rapid and accurate detection methods. Laboratory diagnostics assume greater importance in the absence of clinical manifestations immediately following exposure.
Biological agents. Combinations of diagnostic technologies, such as culture, immunoassay, and molecular assay, can provide rapid diagnostic response capabilities to microbial threats with antimicrobial-resistant organisms, new emerging infectious disease agents, and possible agents of bioterrorism. Conventional microbiology protocols are used at lower-level laboratories, whereas molecular diagnostic assays are used at higher-level laboratories. Polymerase chain reaction-based methods with freeze-dried reagents can be used for quick field detection of microorganisms and bacterial toxins. An ELISA detects circulating toxins. In addition, commercial biosensors are now available for rapid diagnosis of biological agents (eg, Bacillus anthracis and botulinum toxin) (121). Electrochemical biochips enable the detection of chemical and biological warfare agents simultaneously.
The United States Army Medical Research Institute of Infectious Diseases (USAMRIID, Fort Detrick, Maryland) has molecular diagnostic facilities for biological warfare agents. Microarrays using genomic and genetic technologies can reduce the time required for serogroup or strain identification of pathogens affecting the nervous system from days and weeks of cultures to less than a few hours. Although the Institute primarily focuses on protecting military service members, it collaborates with the CDC, the World Health Organization, and academic centers of excellence worldwide.
Anthrax. Treatment should not be delayed pending a diagnostic workup for possible anthrax. Detailed interim guidelines for investigation of, and response to, Bacillus anthracis exposures have been published (35; 36).
No reliable rapid screening test is available to diagnose inhalational anthrax in the early stages (35).
|
• Rapid tests to detect conditions with similar clinical presentations (eg, group A beta-hemolytic streptococcal infections, influenza infections, and respiratory syncytial virus infections in infants) have been encouraged (31; 35). | |
|
• White blood cell counts may be elevated with anthrax (27; 78) but are generally normal (or possibly low) with influenza and other respiratory viral infections. | |
|
• Gram stain and culture of blood, CSF, or pleural fluid can establish the diagnosis of anthrax, but these are late clinical findings. Sputum gram stain is not often positive. | |
|
• Enzyme-linked immunosorbent assay, immunohistology, serum serology (with sequential blood samples at least a week apart), and polymerase chain reaction demonstration of Bacillus anthracis DNA can be used to confirm the diagnosis but are of little help clinically because these typically must be performed at a reference laboratory, and the results are generally not available in time to affect the clinical outcome (88). | |
|
• Nasal swabs can help establish exposure, but anthrax spores are rapidly cleared, which means an exposed person may have a negative swab. Nasal swabs are not indicated to diagnose anthrax, to determine the risk of exposure and need for antimicrobial prophylaxis, or to determine when antimicrobial prophylaxis may be stopped (35; 36). |
With inhalational anthrax, a chest x-ray may demonstrate hilar or mediastinal adenopathy, a widened mediastinum, pleural effusions, or infiltrates (47; 27; 35). Anthrax should be diagnosed if a chest x-ray demonstrates a widened mediastinum in the setting of fever and other constitutional symptoms in the absence of another obvious cause (eg, severe blunt chest trauma or postsurgical infection).
Diagnostic recommendations for anthrax meningitis vary by setting (15).
|
• Conventional (noncrisis) setting. In a conventional (noncrisis) setting, patients with systemic anthrax should undergo a lumbar puncture to determine whether they have anthrax meningitis, provided that no contraindications exist. | |
|
• Contingency setting. In a contingency setting (ie, when resource availability or other circumstances shift evaluation and management to nonpreferred methodologies, medications, and locations), patients with systemic anthrax who have two or more clinical features of meningitis (ie, severe headaches, altered mental status including confusion, meningeal signs, or other neurologic symptoms or signs) or a contraindication to a lumbar puncture should be presumed to have anthrax meningitis, whereas patients with fewer of the screening symptoms and signs should have lumbar punctures. Identification of Gram-positive rods, pleocytosis, visible turbidity, or visible hemorrhage in cerebrospinal fluid is sufficient for diagnosing probable anthrax meningitis. | |
|
• Crisis setting. In a crisis setting (ie, when resource limitations require medical care prioritization, allocating care to those with the highest likelihood of survival or benefit) a clinical case definition (ie, without lumbar puncture) may be used to identify patients with probable anthrax meningitis. Under these circumstances, patients with systemic anthrax and severe headache, altered mental status, meningeal signs, or other neurologic signs should be considered to have anthrax meningitis. |
An evidence-based assessment tool was developed to screen patients for meningitis during an anthrax mass casualty incident (82). Severe headache, altered mental status, meningeal signs, and other neurologic signs at presentation independently predicted meningitis in a derivation cohort. The presence of just one of these signs and symptoms on admission had a sensitivity of 89% for finding anthrax meningitis in the adult validation cohort and 83% in the pediatric validation cohort. Anthrax meningitis was unlikely in the absence of any of these signs or symptoms, whereas anthrax meningitis was very likely in the presence of two or more of these signs or symptoms.
A diagnosis of hemorrhagic meningitis should raise a strong suspicion of anthrax infection (76; 77; 92). In the index case in the 2001 bioterrorist outbreak in the United States, the initial diagnosis was made from CSF (21). Findings are fairly consistent across reported cases (60; 149; 123; 120; 147; 58; 65; 21; 92). CSF in anthrax may appear cloudy, with yellowish or pinkish coloration, or may be grossly bloody; evolution across this spectrum has been reported in serial lumbar punctures in an individual patient. The supernatant is frequently xanthochromic. Opening pressure may be normal or increased (30 to 50 cm of CSF). Hypoglycorrhachia is common, with CSF glucose frequently ranging from 20 to 40 mg/dl. CSF protein is generally elevated, with initial CSF protein values from 120 to 1150 mg/dl (median 400 mg/dl) and with maximum reported values of 1333 mg/dl. CSF is frequently hemorrhagic, but in the early stages, there may be no red cells. There is a polymorphonuclear CSF pleocytosis with a range of 560 to 4750 white cells per cubic mm on initial lumbar puncture (median 2500); the percentage of polymorphonuclear cells ranges from 67% to 95% (median 81%). CSF cultures are usually positive, but CSF may be sterile after several days of antibiotics. Gram stain generally shows numerous polymorphonuclear leukocytes as well as many large gram-positive rods, either singly or in short or long chains; indeed, virtually all case reports of anthrax meningitis that give gram stain results report these findings. Serial lumbar punctures may document an evolution from cloudy to grossly bloody CSF, with increasing protein concentration, numbers of red cells and polymorphonuclear leukocytes, and proportion of polymorphonuclear leukocytes among white cells.
Other laboratory findings in patients with anthrax meningoencephalitis include elevated white blood cell counts (up to 80,000) with a left shift, positive blood cultures in approximately 75%, positive skin biopsy cultures in patients with skin lesions, and frequently abnormal chest x-rays in patients with respiratory symptoms.
Computed tomography or magnetic resonance imaging of the head in patients with anthrax meningoencephalitis may demonstrate focal intracerebral hemorrhage, subarachnoid hemorrhage, intraventricular hemorrhage, diffuse cerebral edema, and prominent leptomeningeal enhancement (58; 86; 66). Parenchymal cerebral enhancement has not been reported (86). Findings may progress rapidly on serial brain imaging studies (58).
Botulism. Like with anthrax, diagnostic recommendations for botulism vary by setting and specifically differ in conventional and contingency settings and crisis settings (125).
When assessing the likelihood of botulism, consider clinical criteria and available epidemiologic data. Classify patients into a botulism likelihood category by clinical judgment. Additional information on clinical criteria is ...
When assessing the likelihood of botulism, consider clinical criteria and available epidemiologic data. Classify patients into a botulism likelihood category by clinical judgment. Additional information on clinical criteria is ...
Laboratory diagnosis of botulism is typically based on the detection of toxin in the patient's serum, stool, or wound. However, because of the minute amount of toxin needed to produce botulism, it may not be possible to recover toxin from tissues or body fluids. Both ELISA and PCR can be used to detect botulinum toxins A and B within 2.5 hours.
Glyco-quantitative PCR enables the detection of active botulinum neurotoxin with high sensitivity (90). Eight known types of botulinum toxin (A through H) can be elaborated by Clostridium botulinum, and knowing which type is present can provide epidemiologic clues regarding the source of exposure (eg, toxin type G does not cause natural disease in humans) (10).
Immunological strategies to screen for botulinum neurotoxin-containing samples include (1) sensitive immunoenzymatic and immunochromatographic assays for fast qualitative and quantitative analyses; (2) a bead-based suspension array is used for screening followed by conventional ELISA for quantification; and (3) an ELISA plate format assay for serotype-specific immunodetection of botulin neurotoxin-cleaved substrates to detect the activity of the light chain rather than the toxin protein (138).
Most physicians are not adequately prepared to diagnose and manage potential bioterrorism agents, including anthrax (48), and not much has changed since the U.S. bioterrorism outbreak in 2001 (143).
Factors that could potentially mitigate the consequences of an anthrax attack include the ability to promptly recognize and manage the illness and its public health consequences, limitation of secondary contamination through appropriate decontamination, and development and implementation of genotyping methods that can facilitate investigations and thereby influence the governmental response to an attack (53). In addition, the circumstances of a significant anthrax outbreak (eg, as in an accidental or intentional release of anthrax spores) could easily overwhelm treatment resources, forcing triage protocols and the use of alternative treatment regimens.
Bacterial biothreat agents. Combining an earlier-generation antibiotic and a broad-spectrum antimicrobial peptide is a useful approach for combating diverse pathogens, including five bacterial biothreats—the etiologic agents of glanders (Burkholderia mallei), melioidosis (Burkholderia pseudomallei), plague (Yersinia pestis), tularemia (Francisella tularensis), and anthrax (Bacillus anthracis) (49). Of these, only anthrax is particularly prone to involve the nervous system.
Anthrax. Naturally occurring Bacillus anthracis is generally susceptible to many antibiotics (28; 92).
For postexposure prophylaxis of inhalational anthrax following intentional exposure to Bacillus anthracis, the CDC recommends initial treatment with ciprofloxacin or doxycycline until antibiotic susceptibility is determined, with total therapy for at least 60 days, in combination with a three-dose series of anthrax vaccine adsorbed administered at time zero, 2 weeks, and 4 weeks (31; 37; 78; 144). Both ciprofloxacin and doxycycline are approved by the U.S. Food and Drug Administration for postexposure prophylaxis of inhalational anthrax in all age groups; ciprofloxacin and doxycycline are considered equivalent first-line agents for postexposure prophylaxis and have similar susceptibility profiles for naturally occurring isolates, similar safety profiles, and a low rate of anaphylactic reactions (144). Long-term use of these antibiotics appears to be relatively safe to treat a large-scale exposure to anthrax in a bioterrorism attack (103). Alternative antimicrobial drugs that can be considered for postexposure prophylaxis if first-line agents are not tolerated or are unavailable include levofloxacin, moxifloxacin, amoxicillin, penicillin VK (if the isolate is penicillin-susceptible), and clindamycin (71).
Anthrax vaccine is now available under an investigational new drug application in combination with extended antibiotic treatment for civilians exposed to inhalational anthrax who have completed a 60-day course of antibiotics (34; 78; 144). A short course of postexposure antibiotic prophylaxis combined with vaccination (ie, initiated simultaneously) has been shown to improve protection against inhalational anthrax in animal models and may shorten the duration of antibiotic prophylaxis needed to protect against inhalational anthrax (158).
There are no controlled trials in humans of potential treatments for inhalational anthrax. Based on studies in nonhuman primates, other animal and in vitro data, and limited clinical experience in people, intravenous ciprofloxacin or doxycycline should be included as essential components of initial therapy until antimicrobial susceptibility is established (28; 31). Ciprofloxacin is the drug of choice in pregnant women (38). Because of the high mortality associated with inhalational anthrax, and because both ciprofloxacin and doxycycline have poor penetration into the CNS, the CDC now recommends combination therapy with at least two drugs that are predicted to be effective. Thus, initial therapy for inhalational (as well as gastrointestinal or oropharyngeal) anthrax should include ciprofloxacin or doxycycline and one or two additional antibiotics with in vitro activity against Bacillus anthracis (eg, ciprofloxacin, rifampin, and vancomycin; ciprofloxacin, rifampin, and clindamycin) (28). The initial dosing of ciprofloxacin in adults is 400 mg intravenously every 12 hours, whereas in children, dosing is 10 to 15 mg/kg every 12 hours (not to exceed 1 gm/d). Initial dosing of doxycycline in adults (or children over 8 years of age and over 45 kg in weight) is 100 mg intravenously every 12 hours, whereas in younger or smaller children, dosing is 2.2 mg/kg intravenously every 12 hours. Initial therapy may be altered based on the clinical course and antimicrobial susceptibility; one or two antibiotics administered orally (eg, ciprofloxacin or doxycycline) may be sufficient if the patient improves (28). Because of the presence of constitutive or inducible beta-lactamases in Bacillus anthracis isolates from the 2001 U.S. outbreak, penicillin alone (eg, penicillin G or amoxicillin) is not recommended as therapy (28; 31; 2001l). Aztreonam, trimethoprim-sulfamethoxazole, or third-generation cephalosporins should not be used. Corticosteroids should be considered as adjunctive therapy for inhalational anthrax associated with extensive edema, respiratory compromise, or meningitis (28). Three doses of the anthrax vaccine can be administered at 0, 2, and 4 weeks, and antibiotics should be continued throughout this period (47; 34; 78). Because respiratory secretions do not spread the disease, standard precautions are adequate.
For anthrax meningitis, ciprofloxacin may be more effective than doxycycline because of better CNS penetration (31; 134), and intravenous ciprofloxacin is, therefore, specifically recommended over doxycycline for treatment of severe systemic or life-threatening anthrax infection (144; 71; 15). Because both ciprofloxacin and doxycycline penetrate poorly into the CSF of patients with meningitis, anthrax meningoencephalitis should be treated with a polydrug antibiotic regimen, utilizing agents that have good CNS penetration in meningitis and provide good antibacterial coverage for Bacillus anthracis for at least 2 to 3 weeks, or until the patient is clinically stable, whichever is longer. Guidelines suggest that empiric treatment for anthrax in which anthrax meningitis is suspected, or cannot be ruled out, should include at least three antimicrobial drugs (71; 15; 118). The preferred regimen includes a fluoroquinolone (ciprofloxacin), a carbapenem (meropenem), and a protein-synthesis inhibitor (linezolid). Intravenous ciprofloxacin is the preferred primary bactericidal component of the multidrug regimen, although levofloxacin and moxifloxacin are considered equivalent alternatives; these fluoroquinolones have adequate CNS penetration and are not known to be susceptible to natural resistance. The carbapenem drugs are highly resistant to beta-lactamases and provide good CNS penetration; meropenem is preferred as the second drug of the combination regimen, although doripenem and imipenem/cilastatin are considered equivalent alternatives. At least one antimicrobial drug that inhibits protein synthesis should also be used to reduce exotoxin production: linezolid is preferred as the first-line protein synthesis inhibitor and is likely to provide better CNS penetration than clindamycin, although toxicity issues must be carefully considered and weighed with linezolid. If meningitis is suspected, doxycycline should not be used because it does not adequately penetrate the CNS.
Although the CDC recommends the use of doxycycline, ciprofloxacin, penicillin G, and amoxicillin for the treatment of human cases and for postexposure prophylaxis to anthrax spores, B anthracis shows a high degree of in vitro susceptibility to many other antimicrobials, suggesting that alternative choices for prophylaxis and therapy may be possible (100). Isolates collected over more than three decades were susceptible to gentamicin, streptomycin, penicillin G, clindamycin, chloramphenicol, vancomycin, linezolid, tetracycline, erythromycin, rifampin, amoxicillin, ciprofloxacin, and doxycycline; the isolates showed intermediate susceptibility to ceftriaxone and cefotaxime but high minimal inhibitory concentration (MIC) values only for trimethoprim.
Corticosteroids have been recommended as an adjunct to the treatment of meningitis in children and are thought to (1) reduce neutrophil migration across the blood-CSF barrier, (2) decrease neutrophil lysosome release in the subarachnoid space, and (3) facilitate reconstitution of the blood-CSF barrier. Steroids have been recommended for consideration as adjunctive agents without any age restriction in anthrax meningitis (28; 71); nevertheless, they should generally be avoided if the antibiotic regimen includes vancomycin because corticosteroids may decrease CSF vancomycin levels (55).
All the recommended antibiotic agents for postexposure prophylaxis or treatment of anthrax may be associated with allergic reactions, and all are likely to cause side effects when administered for 60 days, although the penicillins are likely to be the best tolerated (39). Fluoroquinolones, including ciprofloxacin, can cause various side effects (eg, nausea, vomiting, abdominal pain, diarrhea, anorexia, dizziness, headache, insomnia, mood changes, tremor, restlessness, confusion, and rarely psychosis, hallucinations, seizures, and Achilles tendon rupture). In children, they may cause permanent cartilage damage and arthropathy. Tetracyclines, including doxycycline, frequently cause gastrointestinal disturbances, can cause photosensitivity reactions, and may cause staining and deformity of the teeth in young children (up to age 8 years) or when administered in utero after the fourth month of pregnancy. In pregnant women, doxycycline should be reserved for cases where there are clear contraindications to other appropriate antimicrobial drugs (38). Amoxicillin can cause diarrhea. In a small survey of 490 persons taking postexposure prophylaxis antibiotics in Florida, approximately 20% reported adverse events attributed to the antibiotics after only 1 to 2 weeks, including symptoms of itching, breathing problems, and swelling of the face, neck, or throat (40). Vancomycin requires dosage adjustment and close monitoring in patients with renal failure.
Three anthrax antitoxin antibody preparations were developed with biodefense funding and have received approval from the FDA as adjunctive therapies for inhalational anthrax: raxibacumab, Anthrasil anthrax immune globulin intravenous (AIGIV), and ETI-204 (50). Antitoxin therapy treats the toxemia component of anthrax infection that antibiotics cannot address and that is, in fact, responsible for most of the morbidity and mortality associated with anthrax infection. Despite a lack of human data on these new antitoxins, limited experimental data from animal studies suggest that adjunctive antitoxin therapy may improve survival (75; 50).
Botulism. Rapid diagnosis and multidisciplinary supportive care are the most important components for managing a victim of toxic exposure to botulinum toxin. There is no known cure for the toxic paralysis of botulism. Respiratory support may be required for up to several weeks. In an epidemic of food-borne botulism with respiratory failure and autonomic nervous system involvement, patients receiving botulinum antitoxin within a few days of exposure had a shorter period of ventilator dependency. Unlike the situation with nerve agent intoxication, where there is too much acetylcholine due to inhibition of acetylcholinesterase, the problem in botulism is the lack of the neurotransmitter in the synapse.
Early administration of botulinum antitoxin to neutralize the circulating toxin is important in patients with symptoms that continue to progress. When symptom progression ceases, no circulating toxin remains, and the antitoxin has no effect. Antitoxin may be particularly effective in food-borne cases where, presumably, toxin continues to be absorbed through the gut wall. Treatment of botulism involves the administration of equine-derived heptavalent (A to G) antitoxin, which has been approved by the FDA and is available from the CDC. There is no vaccine against botulinum toxin, although the antitoxin may induce host immunity to the toxin, and, therefore, the antitoxin may be efficacious when used as a vaccine (02).
The effectiveness of Botulism Antitoxin Heptavalent (A, B, C, D, E, F, G), or BAT, is based solely on efficacy studies conducted in animal models of botulism. BAT is made from equine plasma, and hypersensitivity reactions are possible, including infusion reactions, anaphylaxis, and delayed allergic reactions (serum sickness). In clinical trials with healthy volunteers, the most common adverse reactions observed in at least 5% were headache, nausea, pruritus, and urticaria. In a clinical study, the most common adverse reactions reported in at least 1% of patients were pyrexia, rash, chills, nausea, and edema, and only one serious adverse reaction of hemodynamic instability was observed in a single patient.
A systematic review of randomized controlled trials found low- and moderate-certainty evidence supporting the use of intravenous botulinum immune globulin for infant intestinal botulism (45).
Botulin toxin. Botulinum toxin does not cross the placental barrier. Anecdotal reports indicate that women suffering from botulism have borne healthy infants.
All contributors' financial relationships have been reviewed and mitigated to ensure that this and every other article is free from commercial bias.

Douglas J Lanska MD MS MSPH
Dr. Lanska of the University of Wisconsin School of Medicine and Public Health and the Medical College of Wisconsin has no relevant financial relationships to disclose.
See ProfileNearly 3,000 illustrations, including video clips of neurologic disorders.
Every article is reviewed by our esteemed Editorial Board for accuracy and currency.
Full spectrum of neurology in 1,200 comprehensive articles.
Listen to MedLink on the go with Audio versions of each article.
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
General Neurology
Jan. 13, 2025
General Neurology
Jan. 13, 2025
Neuro-Ophthalmology & Neuro-Otology
Jan. 08, 2025
Neuro-Ophthalmology & Neuro-Otology
Jan. 07, 2025
Peripheral Neuropathies
Dec. 30, 2024
General Neurology
Dec. 30, 2024
General Neurology
Dec. 13, 2024
General Neurology
Dec. 13, 2024