Stroke & Vascular Disorders
Neoplastic and infectious aneurysms
Dec. 29, 2024
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Worddefinition
At vero eos et accusamus et iusto odio dignissimos ducimus qui blanditiis praesentium voluptatum deleniti atque corrupti quos dolores et quas.
Intracerebral hemorrhage is much less common than ischemic stroke but is associated with a significantly high mortality and morbidity. Intracerebral hemorrhage frequently affects the basal ganglia, thalamus, cerebral lobes, pons, and cerebellum. Hypertension, cerebral amyloid angiopathy, and anticoagulation are major causes of intracerebral hemorrhage. The cerebellum has emerged as a frequent location of coagulant-associated intracranial hemorrhage. Statins are associated with only a marginal risk of intracerebral hemorrhage. Non-vitamin K antagonist oral anticoagulants are associated with lesser frequency of intracerebral hemorrhage to that of smaller size. Alcohol consumption in moderate amounts decreases the risk of both lobar and nonlobar intracerebral hemorrhage. Carriers of apolipoprotein E2 and E4 have an increased risk of intracerebral hemorrhage in lobar locations, presumably because of the effects of these gene variants on risk of cerebral amyloid angiopathy. Genetic studies identify 1q22 as a susceptibility locus for intracerebral hemorrhage. A “Causal Classification System for Intracerebral Hemorrhage” divides intracerebral hemorrhages in five subtypes. These include arteriolosclerosis, cerebral amyloid angiopathy, mixed small vessel disease, other rare forms of small vessel disease (genetic), and secondary causes (macrovascular, tumor, and other rare causes). Hematoma expansion is an accurate predictor of poor outcome of intracerebral hemorrhage. "Spot sign" on CT angiography has been reported to predict hematoma expansion. Strict blood pressure control may prevent further enlargement of hematoma. Anticoagulation-related intracerebral hemorrhage is often fatal, and rapid reversal of anticoagulation is the most effective therapy currently available. Severe COVID-19 is often associated with marked coagulopathy predisposing an intracerebral hemorrhage. Intracerebral hemorrhage can be a devastating complication of COVID-19. Vaccine-induced immune thrombocytopenia and thrombosis is characterized by immune-mediated thrombocytopenia and cerebral venous thrombosis and cerebral hemorrhage following the ChAdOx1 nCoV-19 adenoviral vector vaccine administration. It is associated with high mortality. Levetiracetam is a preferred drug for seizure control as it has a neuroprotective effect against posthemorrhagic stroke brain injury. Surgical evacuation of hematoma for supratentorial intracerebral hemorrhage was not shown to be beneficial. Considering a high rate of early neurologic deterioration in the first few hours, the American Heart Association recommends identifying patients at high risk of hematoma expansion. The FAST-MAG trial suggested that magnesium sulfate might reduce hematoma growth and neurologic decline in stroke patients, but further research is necessary to confirm these preliminary findings. In this article, the author reviews the different aspects of intracerebral hemorrhage in detail.
|
• Intracerebral hemorrhage is a common cause of stroke. | |
|
• It results from hypertensive damage to blood vessels, rupture of an aneurysm or arteriovenous malformations, cerebral amyloid angiopathy, altered hemostasis (like thrombolysis and anticoagulation), hemorrhagic necrosis (like tumor and infection), or substance abuse (cocaine). | |
|
• Common sites for involvement include the basal ganglia, lobes of cerebral hemispheres, thalamus, pons, cerebellum, and other brainstem sites. | |
|
• Computed tomography readily demonstrates acute hemorrhage. | |
|
• Initial management is focused on maintaining breathing, circulation, and fluid and electrolyte balance. | |
|
• Quick hemostasis to prevent hematoma expansion, surgical removal of clots, removal of intraventricular blood, and prompt blood pressure control improve outcomes. |
Nontraumatic intracranial hemorrhage refers to bleeding into the substance of the brain in the absence of trauma or surgery. It includes intracerebral (intraparenchymal), subarachnoid, epidural, and subdural hemorrhage. Parenchymal intracerebral hemorrhage represents approximately 10% of all strokes and two thirds of hemorrhagic strokes. This article will focus primarily on parenchymal intracerebral hemorrhage.
Intracerebral hemorrhage is characterized by a sudden onset of focal neurologic deficit progressing over hours along with headache, nausea, vomiting, altered consciousness, and hypertension. Clinical manifestations depend on hematoma location. Patients with supratentorial hemorrhage often present with contralateral hemiplegia, hemisensory loss, aphasia, neglect, gaze abnormalities, and hemianopia. Infratentorial hemorrhages manifest with signs of brainstem dysfunction, cranial nerve abnormalities, ataxia, nystagmus, and other cerebellar signs.
Conjugate eye deviation frequently occurs in patients with supratentorial intracerebral hemorrhage. Conjugate eye deviation can be evoked by a relatively smaller thalamic hematoma than a putaminal hematoma. In a study, the persistence of conjugate eye deviation was a significant predictor of death (137).
Seizures occur in approximately 10% of all patients with intracerebral hemorrhage and in almost one half of patients with lobar hemorrhage. Often seizures occur at the onset of bleeding or within the first 24 hours of an acute event. Posthemorrhagic seizures, possibly nonconvulsive, have been associated with neurologic worsening on the NIH Stroke Scale and with an increase in midline shift (155). In one series, seizures were reported to occur in one third of patients with intracerebral hemorrhage (31). In over half of these patients, the seizures were purely electrographic. Electrographic seizures were associated with expanding hemorrhages, and periodic discharges were associated with cortical intracerebral hemorrhage and poor outcomes. Another study demonstrated that the mean intracerebral hemorrhage volume was independently associated with seizures, and an increase of 1 mm3 in hematoma volume increased the seizure rate by 2.7% (172).
Early delirium is a frequent and serious complication of intracerebral hemorrhage and is associated with high mortality. Marrama and colleagues observed that preexisting dementia, heavy alcohol intake, and lobar location of hemorrhage independently predicted early-onset delirium among patients with intracerebral hemorrhage (100).
Blood may rupture into the ventricles and cause hydrocephalus. Rarely, blood may also leak into the subarachnoid space. A large hematoma may compress over adjacent structures such as the brainstem and thalamus, causing herniation and death. There can be a rapid rise in intracranial pressure, leading to rapid deterioration of sensorium within a few minutes or hours. Such patients often present with coma, bilateral plantar extensor reflexes, pupillary abnormalities, or Cheyne-Strokes breathing indicating the development of brain herniation. If hemorrhage continues worsening, death may result because of compression of vital brainstem centers.
Basal ganglionic capsular hemorrhage. Anatomically, basal ganglia include the putamen, globus pallidus, and caudate nucleus. These structures are supplied by different arteries. Therefore, striatocapsular hemorrhage can be subdivided into specific types according to arterial involvement (30). Putaminal hemorrhage is the most common type of hemorrhage secondary to hypertension. Contralateral hemiparesis, hemisensory loss, or hemi-inattention is generally present. Aphasia is frequently seen if hemorrhage occurs in the posterior limb of the left internal capsule (87). Transient conjugate eye deviation to the lesion side can occur if the globus pallidus or medial putamen is involved.
Massive putaminal hemorrhage can extend into the ventricle or herniate onto the brainstem. When deterioration in consciousness occurs in patients with putaminal hemorrhage, hematoma enlargement is more likely to occur than in intraventricular extension (71). As the lesion enlarges, the ipsilateral pupil first becomes smaller and later larger than a normal pupil; ipsilateral plantar response becomes extensor; and bilateral horizontal gaze palsy develops.
Caudate hemorrhage is frequently associated with intraventricular extension and secondary hydrocephalus. Caudate hemorrhage causes severe headaches, nausea, vomiting, and signs of meningeal irritation. Motor focal neurologic deficit develops when the hematoma is large enough to involve the putamen and internal capsule (76).
Thalamic hemorrhage. The thalamus is a structure with multiple nuclei and functions. It serves as a relay center for sensory signals prior to their final pathways to the parietal lobe and also contains nuclei that are responsible for wakefulness. Approximately 15% of all hypertensive hemorrhages are thalamic hemorrhages. Hemisensory loss, decreased sensorium, and hemiparesis are usual manifestations of thalamic hemorrhage. Intraventricular extension is more commonly seen than in putaminal hemorrhage and causes obstructive hydrocephalus and, in turn, stupor or coma (144). Pure sensory deficits are more commonly seen in thalamic infarction than in hemorrhage. Cognitive dysfunction may include aphasia, neglect, and anosognosia. Vertical gaze abnormalities, upgaze palsy, downward tonic deviation of gaze, small fixed and sluggish pupils, are characteristic neuro-ophthalmological manifestations in thalamic hemorrhage.
Pontine hemorrhage. Pontine hemorrhages typically present with rapid onset of coma, pinpoint pupils, disturbed respiratory patterns, autonomic instability, quadriplegia, and horizontal gaze paralysis. Occasionally, ocular bobbing can also be seen as well as miotic pupils.
Damage of the upper pontine reticular formation is responsible for abrupt coma. Quadriparesis is not uncommon especially when hemorrhage occurs at the motor fiber decussation. Although narcotic overdose may present with abrupt coma and miotic pupils mimicking pontine hemorrhage, other cranial nerve function generally remains intact.
Cerebellar hemorrhage. Like other hypertension-related hemorrhages, cerebellar hemorrhage is typically located in the area of the dentate nucleus, which is supplied by small penetrating vessels with microscopic hypertensive changes. The most common symptoms of cerebellar hemorrhage are vertigo, severe nausea and vomiting, and ataxia. Headache may be severe. Patients with cerebellar hemorrhage can rapidly become comatose within hours after the onset. Alteration of mental status can be secondary to damage to the pons or midbrain or abrupt obstructive hydrocephalus. Occasionally, peripheral facial weakness and horizontal gaze impairment can also occur, representing herniation onto the pons. Cerebellar clots greater than 3 cm in diameter have a poor prognosis if left untreated. In a study examining cerebellar intracerebral hemorrhage, superficial cerebellar intracerebral hemorrhage was found to be strongly associated with cerebral amyloid angiopathy, whereas deep/mixed cerebellar intracerebral hemorrhage correlated with hypertensive arteriopathy. Analyzing 197 patients using the latest Boston criteria, this relationship was evident through various imaging markers and clinical features, such as cerebral microbleeds and systolic blood pressure, reinforcing the significance of cerebellar intracerebral hemorrhage topography in determining its underlying etiology (67).
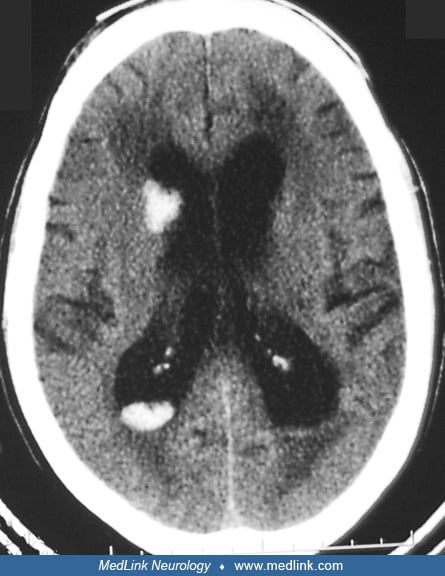
Lobar hemorrhage. This is one of the most clinically silent hemorrhages; brain imaging is of utmost importance in making the diagnosis. Clinical presentations depend on the location and size of the hematoma, varying from no symptoms at all to focal neurologic deficits corresponding to the area of the brain being damaged. Hematoma in the frontal lobe may present as abulia, aphasia, contralateral hemiplegia, and conjugate eye deviation toward the side of the hemorrhage. Parietal hematoma manifests as hemisensory loss, visual inattention, and abnormalities of visuospatial functions. If hematoma involves the inferior parietal region, the patient may have abnormalities in reading, writing, and calculation. Occipital hematoma presents with hemianopia. Temporal hematoma manifests as delirium and Wernicke aphasia. Large hematomas may produce coma if they compress over the midbrain and thalamus.
In patients with cerebral amyloid angiopathy-related lobar intracerebral hemorrhage, the parietal lobes are the most frequently affected site (62). Lobar cerebral bleeds alone are generally associated with cerebral amyloid angiopathy whereas concurrent lobar and deep microbleeds suggest hypertensive angiopathy (177). Recurrence of lobar intracerebral hemorrhage is associated with previous microbleeds or macrobleeds and posterior CT white matter hypodensity, which may be markers of severity for underlying cerebral amyloid angiopathy. Use of an antiplatelet agent following lobar intracerebral hemorrhage may also increase recurrence risk (16). Patients of lobar intracerebral hemorrhage have substantially high risks for recurrent stroke, dementia, and lower quality of life (84).
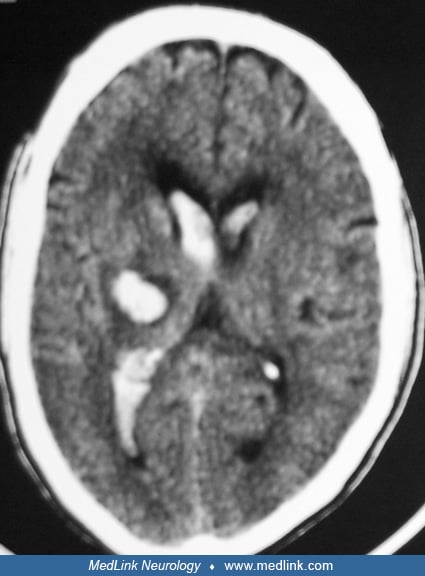
Intraventricular hemorrhage. Primary intraventricular hemorrhage is a rare form of intracerebral hemorrhage characterized by direct bleeding into the ventricular system of the brain. Primary intraventricular hemorrhage accounts for only 3% of all spontaneous intracerebral hemorrhages. Hypertension is a major cause of primary intraventricular hemorrhage. Short-term outcomes depend on patient age and the extent of intraventricular hemorrhage. The yield of diagnostic cerebral angiography in the setting of primary intraventricular hemorrhage is very high. The two most common causes identified on angiography are arteriovenous malformations and aneurysms (45).
Intraventricular hemorrhage can occur following rupture of parenchymal hemorrhage into the ventricular system or can result from primary diseases of the ventricular system of the brain or structures just beneath the ventricular wall. Thalamic and caudate locations had the highest intraventricular hemorrhage frequency (69% and 100%) (54). This condition is clinically characterized by sudden onset of severe headache, vomiting, progressive deterioration in consciousness, and signs of meningeal irritation. If the hemorrhage is massive, signs of brainstem herniation are present. Most of these patients have associated hydrocephalus, and some may require ventricular drainage. The two most common causes of primary intraventricular hemorrhage identified by angiography are arteriovenous malformations and aneurysms (45). Anticoagulant administration increases intraventricular hemorrhage volume and risk of intraventricular extension in lobar and deep intracerebral hemorrhage (15). Patients with intraventricular hemorrhage are twice as likely to have a poor outcome when compared to patients without intraventricular hemorrhage. Caudate location has been associated with a good outcome despite 100% incidence of intraventricular hemorrhage (54). Approximately one third of patients with primary intraventricular hemorrhage do not survive hospital discharge. Patient age and amount of intraventricular hemorrhage independently predict in-hospital mortality (45). A new intraventricular hemorrhage or an intraventricular hemorrhage expansion predicts poor outcome (174). Intraventricular thrombolysis in patients with intraventricular hemorrhage may be associated with better outcome (38).
Multiple intracerebral hemorrhages. Simultaneous occurrence of multiple intracerebral hemorrhages in different arterial territories is a rare clinical event. Multiple simultaneous intracerebral hemorrhages account for 5.6% of all spontaneous intracerebral hemorrhage (145). Causes of multiple intracerebral hemorrhages include cerebral amyloid angiopathy, venous sinus thrombosis, coagulopathy, vasculitis, hemorrhagic transformation of cerebral infarcts, and multiple intracranial pathologies such as vascular anomalies or tumors. Although hypertension is the most common etiologic factor for the development of spontaneous single intracerebral bleeding, its role in simultaneous multiple intracerebral hemorrhages is not clear (173).
Hemorrhagic infarct. Pregnancy and the puerperium can be associated with cerebral hemorrhage due to cerebral venous thrombosis and eclampsia. In cerebral venous thrombosis, the affected cortex and underlying white matter becomes congested, swollen, and hemorrhagic, leading to venous infarction. Hemorrhage in the venous infarct range from a large hematoma to petechial hemorrhages within the infarct.
Vaccine-induced immune thrombocytopenia and thrombosis (VITT). Vaccine-induced immune thrombocytopenia and thrombosis is characterized by immune-mediated thrombocytopenia and a widespread venous thrombosis following the ChAdOx1 nCoV-19 adenoviral vector vaccine administration. Cerebral venous thrombosis is the most serious form of thrombosis in VITT. There is a very high risk of mortality if VITT is associated with cerebral venous thrombosis and intracerebral hemorrhage. A high titer of platelet factor-4 antibodies is demonstrated, and these antibodies activate platelets. D-dimer levels are generally markedly elevated (higher than 4000 FEU). Immunotherapy is helpful in many cases (124).
In a risk factor analysis, age, African-American ethnicity (vs. Caucasian), and hypertension were positively associated with the occurrence of intracerebral hemorrhage, whereas low-density lipoprotein cholesterol and triglycerides were inversely related to the intracerebral hemorrhage (146). Patients with a systolic blood pressure of 160 mm Hg or higher or diastolic blood pressure of 110 mm Hg or higher had 5.55 times the rate of intracerebral hemorrhage as nonhypertensives. Sex, smoking, alcohol intake, body mass index, waist-to-hip ratio, waist circumference, and diabetes were not related to intracerebral hemorrhage. Hypertension significantly increases the risk of intracerebral hemorrhage, particularly in persons who have poorly controlled hypertension. Advancing age, smoking, and excessive use of alcohol also increase the risk of intracerebral hemorrhage. Alcohol consumption in moderate amounts decreases the risk of both lobar and nonlobar intracerebral hemorrhage (27).
Vascular changes initiated by hypertension are responsible for primary intracerebral hemorrhage in the majority of patients. Cerebral amyloid angiopathy is the other major cause of primary intracerebral hemorrhage and an important cause of lobar hemorrhage in elderly populations. Cerebral amyloid angiopathy represents beta-amyloid deposition in the small- and medium-sized vessels of the brain and meninges. Cerebral amyloid angiopathy plays a major role in the pathogenesis of intracerebral hemorrhage even in patients with more evident risk factors like hypertension. Hereditary intracerebral hemorrhage with amyloidosis, Dutch type, is a rare, autosomal-dominant type of cerebral amyloid angiopathy that results in recurrent, sometimes multiple, lobar hemorrhages.
Genetic studies identify 1q22 as a susceptibility locus for intracerebral hemorrhage. In a cohort of 1545 patients with intracerebral hemorrhage (664 lobar and 881 nonlobar cases) and 1481 controls, investigators identified two susceptibility loci: chromosomal region 12q21.1 (rs11179580) for lobar intracerebral hemorrhage and chromosomal region 1q22 (rs2984613) for nonlobar intracerebral hemorrhage (164). The apolipoprotein E polymorphism is a risk factor for intracerebral hemorrhage. This risk is related to cerebral amyloid angiopathy in the white population. An observation noted that among carriers of the epsilon2 or epsilon4 allele of the apolipoprotein E polymorphism, Asians have a greater risk of intracerebral hemorrhage than Europeans (153). Carriers of apolipoprotein E2 and E4 have an increased risk of intracerebral hemorrhage in lobar locations, presumably because of the effects of these gene variants on the risk of cerebral amyloid angiopathy. It has been suggested that vasculopathic changes associated with the apolipoprotein E2 allele might have a role in the severity and clinical course of lobar intracerebral hemorrhage (14). A group of authors identified frequent collagen, type IV, alpha 1 (col4a1) gene mutations in patients with sporadic intracerebral hemorrhage (161). Data support the hypothesis that increased intracellular accumulation of collagen, type IV, alpha 1, decreased extracellular collagen, type IV, alpha 1, or both, contribute to sporadic cerebrovascular disease and intracerebral hemorrhage. An investigation revealed that apolipoprotein E4 is associated with recurrent intracerebral hemorrhage in nonlobar brain regions as well (128).
Several studies observed that higher levels of cholesterol were associated with a lower risk of intracerebral hemorrhage. A higher level of low-density lipoprotein cholesterol also seems to be associated with a lower risk of hemorrhagic stroke, whereas a high-density lipoprotein cholesterol level seems to be positively associated with risk of intracerebral hemorrhage (159). In a study, low serum triglyceride levels were associated with an increased risk of intracerebral hemorrhage and with the presence of deep or infratentorial cerebral microbleeds (162). However, the authors did not observe an increased risk of intracerebral hemorrhage with the widespread use of lipid-lowering statins in the population (52). A meta-analysis also suggests that statin use before spontaneous intracerebral hemorrhage does not increase short-term mortality, unfavorable functional outcome, or hematoma volume (83). Another meta-analysis suggests that irrespective of stroke subtype, there is only marginal risk of future intracerebral hemorrhage with statins (184). An investigation revealed that lipid levels differ between intracerebral hemorrhage cases and non-intracerebral hemorrhage controls. Authors observed a decline in serum total cholesterol and low-density lipoprotein levels within 6 months preceding primary intracerebral hemorrhage, independent of statin or alcohol use (125).
Anticoagulation is also associated with a substantial risk of intracerebral hemorrhage, with an estimated 9% to 14% of all intracerebral hemorrhage attributable to antithrombotic therapy. Anticoagulants not only increase the risk but also increase the severity of intracerebral hemorrhage by causing hematoma expansion. Cerebellar location is significantly more common in coagulant-associated intracerebral hemorrhage (138). The mortality rate is about 65%, and most of the surviving patients remain disabled. Intracerebral hemorrhage rates range from 0.3% to 0.6% per year during oral anticoagulation. Risk factors for anticoagulation-associated intracerebral hemorrhage include international normalized ratio values greater than 3, previous cerebrovascular disease, and CT findings suggestive of leukoaraiosis. Antiplatelet agents with anticoagulation and the combined use of aspirin plus clopidogrel further increase the risk of intracerebral hemorrhage. However, modestly lowering blood pressure significantly reduces the risk of intracerebral hemorrhage during antiplatelet therapy (58). A Cochrane Review update evaluated the effectiveness and safety of antithrombotic treatments post-intracerebral hemorrhage (32). Analyzing nine randomized controlled trials with 1491 participants, it found no clear evidence of benefits or risks from short-term prophylactic anticoagulation or long-term oral antiplatelet therapy post-intracerebral hemorrhage. Long-term therapeutic dose oral anticoagulation for atrial fibrillation post-intracerebral hemorrhage may reduce major adverse cardiovascular events and occlusive vascular events but potentially increases intracranial hemorrhage.
Patients using non-vitamin K antagonist oral anticoagulants (rivaroxaban, apixaban, and edoxaban or dabigatran) have a lesser frequency of intracerebral hemorrhage in comparison to that with warfarin. Among 141,311 patients with intracerebral hemorrhage, 15,036 (10.6%) were taking warfarin and 4918 (3.5%) were taking non-vitamin K antagonist oral anticoagulants preceding intracerebral hemorrhage, and 39,585 (28.0%) and 5783 (4.1%) were taking concomitant single and dual antiplatelet agents, respectively (68). Non-vitamin K antagonist oral anticoagulants-related intracerebral hemorrhage is associated with smaller hematoma and lesser neurologic deficit compared to vitamin K antagonist-related intracerebral hemorrhage (152).
Intracerebral hemorrhage is the most serious complication of fibrinolytic therapy for acute myocardial infarction, pulmonary embolism, and ischemic stroke. There is increasing evidence that cerebral amyloid angiopathy, which itself can cause hemorrhage, may be a risk factor for thrombolysis-related intracerebral hemorrhage. Cerebral amyloid angiopathy and thrombolysis-related intracerebral hemorrhage share some clinical features, such as predisposition to lobar or superficial regions of the brain, multiple hemorrhages, increasing frequency with age, and an association with dementia (105).
Sympathomimetic drugs have long been associated with intracerebral hemorrhage. Intracranial hemorrhage due to cocaine, amphetamine, or phenylpropanolamine use may be secondary to sudden elevation in blood pressure, multifocal cerebral vessel spasm, or drug-induced vasculitis. Since 1979, several case reports have documented the relationship between phenylpropanolamine (a common constituent of cough syrups and over-the-counter anti-cold medications) and stroke.
Aneurysms, arteriovenous malformations, cavernous angiomas, dural arteriovenous fistulas, and venous malformations all can result in intracerebral hemorrhage. The hemorrhage due to a ruptured aneurysm almost always has a subarachnoid component and often extends into the ventricles. The predictors of hemorrhage in arteriovenous malformation include increasing age, deep brain location, and exclusive deep venous drainage. The risk of spontaneous hemorrhage may be low in patients with arteriovenous malformations without these risk factors (142).
Spontaneous intracerebral hemorrhage is not an uncommon presentation in cases of previously unsuspected brain tumors. Hemorrhage resulting from brain tumors can occur in up to 10% of all primary or metastatic tumors. Hemorrhage is more likely with certain types of tumors, including glioblastoma, hemangioblastoma, oligodendroglioma, and metastatic tumors. Metastatic tumors with a high propensity to hemorrhage are malignant melanoma as well as renal cell, prostate, and lung cancer. Choriocarcinomas are malignant neoplastic tumors from the trophoblastic tissue with a tendency to early metastases. Aside from pulmonary metastases, there are often cerebral metastases, leading to intracerebral hemorrhage often responsible for the first clinical symptoms.
|
Hypertension | |
|
Hematologic disorders | |
|
(1) Platelet disorders: thrombocytopenia, platelet dysfunction | |
|
Blood vessel abnormalities | |
|
(1) Arteriovenous malformation | |
|
Hemorrhagic infarct in cerebral venous thrombosis | |
|
Hemorrhagic transformation of a cerebral infarction | |
|
Tumors | |
|
(1) Primary brain tumor: much less common than metastatic tumor | |
|
Drug-induced causes | |
|
(1) Illicit drugs: cocaine, amphetamine | |
COVID-19 and intracerebral hemorrhage. The pandemic COVID-19, caused by SARS-COV-2 virus, is dominantly characterized by lung involvement and acute respiratory failure in serious cases. Severe COVID-19 is often associated with marked coagulopathy with abnormalities in certain coagulation parameters. Typical COVID-19-associated coagulopathy is characterized by raised D-dimer levels, elevated fibrinogen levels, thrombocytopenia, and raised partial thromboplastin time and activated partial thromboplastin time. COVID-19 associated with hypercoagulability often needs to be treated with systemic anticoagulation. Intracerebral hemorrhage can be a devastating complication of COVID-19 (13). A meta-analysis analyzed all kinds of strokes that were seen in COVID-19 and noted that out of 17,799 COVID-19 patients, 156 (0.9%) patients had a stroke, and 27 (17%) patients had intracerebral/subarachnoid hemorrhage (139). Melmed and colleagues reviewed neuroimaging data of 755 patients with COVID-19; intracerebral hemorrhage was recorded in 33 (7.9%) patients (106). Older age, non-Caucasian race, respiratory failure requiring mechanical ventilation, and systemic anticoagulation were significant predictors of intracerebral hemorrhage. Anticoagulation use was associated with a 5-fold increased risk of intracerebral hemorrhage. Frequently in COVID-19, patients have multiple hematomas with frequent intraventricular extension (29). Use of extracorporeal membrane oxygenation therapy significantly enhances the risk of multiple hemorrhages in COVID-19 (17). In severe COVID-19, intracerebral microhemorrhages may be part of diffuse leukoencephalopathy and extensive white matter necrosis.
Raposo and colleagues devised a “Causal Classification System for Intracerebral Hemorrhage” subtypes. In this classification system, five intracerebral hemorrhage subtypes have been categorized; these include arteriolosclerosis, cerebral amyloid angiopathy, mixed small vessel disease, other rare forms of small vessel disease (genetic small vessel disease and others), and secondary causes (macrovascular causes, tumor, and other rare causes) (129).
Hypertensive vascular changes in the deep perforating small arteries are characterized by the proliferation of arteriolar smooth muscles, followed later by apoptotic smooth muscle cell death and collagen deposition. This sequence of degenerative changes results in the replacement of smooth muscle cells in the tunica media with collagen fibers. The hyalinization process increases the fragility of cerebral vessels. Intracerebral hemorrhage occurs following the rupture of fragile vessels within the cerebral parenchyma.
Cerebral amyloid angiopathy is caused by the deposition of amyloid alpha protein within the adventitia and media of leptomeningeal and parenchymal arteries. In the arteries affected by cerebral amyloid angiopathy, local muscle and elastic elements are replaced by amyloid fibrils, thereby weakening the wall of the vessel. Brittleness of arterioles with poor contractile capability is possibly responsible for the occurrence of hemorrhage and early growth of hematomas after rupture. It has been suggested that because arteriolar bleeding is slower than arterial bleeding, it is possible to intervene to stop ongoing hematoma expansion. For example, recombinant factor VIIa or other therapies may be useful (07).
Cerebral microbleeds detected by gradient-echo MRI are considered evidence of advanced microangiopathy with potential for further bleeding. These dot-like, low-intensity spots (dot-like hemosiderin spots) on T2-weighted MRI images have been histologically diagnosed as old cerebral microbleeds.
Cerebral microbleeds might indicate a higher risk of future intracerebral hemorrhage and may be a marker of cerebral small-vessel disease and cerebral amyloid angiopathy. Primary intracerebral hemorrhage without microbleeds is more common in younger patients with precipitating events, whereas intracerebral hemorrhage with microbleeds are more common in elderly patients with prominent ischemic change and frequent use of antithrombotics or anticoagulants (70). Asian patients with intracerebral hemorrhage are more likely to have cerebral microbleeds than non-Asian patients. In cerebral amyloid angiopathy, microhemorrhages predict the risk of recurrent lobar intracerebral hemorrhage (156). Lee and colleagues performed a radiologic study on the cerebral microbleeds and volume of intracerebral hemorrhage in patients with supratentorial intracerebral hemorrhage (Lee at al 2006). Of the patients with lobar or putaminal hemorrhage, the hemorrhage volumes increased more than 2-fold or 3-fold in the patients with microbleeds. Moreover, the presence of microbleeds was an independent risk factor for large-sized hemorrhage. The number of initial microbleeds on baseline gradient-echo T2-weighted MR imaging is significantly associated with development of recurrent intracerebral hemorrhage and may predict the recurrence of the intracerebral hemorrhage (69). Leukoaraiosis is a risk factor for symptomatic intracerebral hemorrhage after thrombolysis for acute stroke (119).
A substantial amount of neuronal damage and development of edema often leads to clinical deterioration after intracerebral hemorrhage. The mechanisms of perihematomal neuronal injury in intracerebral hemorrhage are not completely understood. Inflammatory mediators from the blood enter into the brain parenchyma and induce an inflammatory reaction, although the brain cells themselves are capable of producing many of these inflammatory changes. The coagulation cascade is activated as soon as blood comes in contact with neuronal tissue. Thrombin, heme oxygenase, complement, microglia activation, and leukocyte infiltration all have been shown to be involved in the pathogenesis of intracerebral hemorrhage (72). Matrix metalloproteinases are a family of proteases involved in the remodeling of the extracellular matrix. In the central nervous system, matrix metalloproteinases are involved in the mechanisms associated with inflammation. The role of matrix metalloproteinases in hemorrhagic stroke appears critical for hematoma and brain edema growth as well as for neuronal death, which are understood as secondary brain injury and may have a considerable clinical impact (46). Matrix metalloproteinases -9 have been shown to induce a high breakdown capacity, especially in the arteriolar basement membrane, leading to cerebral edema and secondary hemorrhage. Glutamate is accumulated in abundance during the early period of experimental hematoma, and the activation of NMDA receptors by glutamate can result in an influx of calcium and neuronal death in cases of intracerebral hemorrhage (07; 168).
Perihematomal edema volume increases by approximately 75% during the first 24 hours after hyperacute spontaneous intracerebral hemorrhage. Patients with the least amounts of baseline relative edema volume were most likely to develop significant additional amounts of edema during the first 24 hours after spontaneous intracerebral hemorrhage (48). Early edema is probably related to the release of osmotic substances from the clot rather than the impairment of the blood-brain barrier. Brain edema develops in response to clot retraction, thrombin formation, erythrocyte lysis, hemoglobin toxicity, complement activation, mass effect, and blood-brain barrier disruption. It has been demonstrated that early hematoma evacuation interrupts edema formation (167; 63; 131). Factors associated with larger edema growth are older age, higher NIHS score, lower Glasgow Coma scale score, larger hematoma volume, larger initial edema, irregularly shaped hematoma, and higher glucose level. Patients with faster edema growth had more midline shift, herniation, and higher 6-month mortality. In the logistic regression analysis, higher-than-expected edema growth was associated with 6-month mortality (166). Sprügel and colleagues noted that the shape of the hematoma also acted as a determinant of peak perihemorrhagic edema (141). In patients with irregularly shaped hematoma, perihemorrhagic edema was larger, and these patients may benefit from antiedema measures.
The intracerebral hemorrhage core is primarily a collection of red blood cells. Often it is possible to recognize fragments of torn vessels at the periphery of intracerebral hemorrhage. Perihematomal microscopic pathology comprises fragments of necrotic brain tissue with neutrophils and macrophages around the damaged area as early as 2 days after the onset. Macrophages ingest degraded red blood cells, hemosiderin, and disintegrated myelin. Red blood cells begin to lyse in 1 week, with sequential changes of hemoglobin to deoxyhemoglobin and methemoglobin. Astrocytic proliferation appears in a later stage and ends with glial fibril formation. Phagocytosis is necessary to eliminate the hematoma after intracerebral hemorrhage; however, the release of proinflammatory mediators and free radicals during phagocyte activation is toxic to neighboring neuronal cells, leading to secondary brain injury. It has been suggested that enhancement of phagocytosis may limit the toxic effects of persistent blood products on surrounding neuronal tissue. Microglia isolated from murine brains showed more efficient phagocytosis in response to peroxisome proliferator-activated receptor gamma activators. An experimental study demonstrated that the peroxisome proliferator-activated receptor gamma agonist, rosiglitazone, promoted hematoma resolution, decreased neuronal damage, and improved functional recovery in a mouse intracerebral hemorrhage model (180). In another experimental study, authors observed that toll-like receptor 4-deficient-mice had markedly decreased perihematomal inflammation, associated with reduced recruitment of neutrophils and monocytes, fewer microglia, and improved functional outcome (136).
Early reperfusion after an ischemic stroke can cause blood-brain barrier injury with subsequent cerebral edema and devastating brain hemorrhage (97).
Each year in the United States, approximately 37,000 to 52,400 people suffer an intracerebral hemorrhage. This figure accounts for approximately 10% of all strokes. The number of patients with intracerebral hemorrhage is expected to increase substantially over the next few decades because of the increasing age of the population (64; 20; 134; 53). A significant rise in rates of intracerebral hemorrhage and a decrease in rates of cerebral infarction were noted in Quebec, Canada over 15 years. The decrease in the rates of cerebral infarction was 32.5% for men and 25.5% for women. A concomitant increase in rates of intracerebral hemorrhage was 28% for men and 22% for women (104). The incidence of anticoagulant-associated intracerebral hemorrhage quintupled in our population during the 1990s. The majority of this change can be explained by increasing warfarin use. Anticoagulant-associated intracerebral hemorrhage now occurs at a frequency comparable to subarachnoid hemorrhage (42). UK stroke mortality data, however, suggest that the incidence of hemorrhagic stroke has fallen in the past 20 years. Data from the Oxford Community Stroke Project and the Oxford Vascular Study suggested that in the population aged less than 75 years the incidence of intracerebral hemorrhage decreased substantially. However, above 75 years of age, the proportion of intracerebral hemorrhage cases that were nonhypertensive with lobar bleeds and presumed to have had mainly amyloid-related hemorrhages increased (93). After analyzing a nationwide inpatient sample (2004-2018) and U.S. Census Bureau data, Bako and colleagues noted an increased 3-year incidence (per 100,000 population) of intracerebral hemorrhage from 62.79 during 2004 to 2006 to 78.86 during 2016 to 2018 (10). The increased incidence of intracerebral hemorrhage was much faster in the younger population and coincided with an increased prevalence of hypertension and anticoagulant use.
A marked racial variation in the incidence of intracerebral hemorrhage has been observed. Mexican Americans have higher incidence rates of intracerebral hemorrhage than non-Hispanic whites. There are significant differences in the characteristics of intracerebral hemorrhage in Mexican Americans and non-Hispanic whites, with Mexican American patients more likely to have smaller, nonlobar hemorrhages (178). Intracerebral hemorrhage is much more common in Asian populations as well, probably reflecting higher rates of small vessel disease, hypertension, and genetic factors. A family history of intracerebral hemorrhage appears to be a significant risk factor for nonlobar intracerebral hemorrhage at ages above 70 years. The presence of Apo E4 appears to be a risk factor for lobar intracerebral hemorrhage at age 70 years or older but not at ages younger than 70 years (165). Men are more likely to suffer an intracerebral hemorrhage than women.
In a population-based study from Sweden, risk factors for primary intracerebral hemorrhage and primary intracerebral hemorrhage subtypes were investigated in 22,444 men and 10,902 women. In this study, 147 subjects with CT- or autopsy-verified, first-ever primary intracerebral hemorrhage were compared with 1029 stroke-free controls. High systolic blood pressure, diabetes, high triglycerides, short stature, and psychiatric morbidity remained significantly associated with primary intracerebral hemorrhage. High systolic blood pressure was significantly associated both with nonlobar and lobar primary intracerebral hemorrhage. Diabetes and psychiatric morbidity were associated with nonlobar primary intracerebral hemorrhage. Smoking doubled the risk for lobar primary intracerebral hemorrhage but was unrelated to nonlobar primary intracerebral hemorrhage (182).
On the contrary, Asian countries have a higher incidence of intracerebral hemorrhage than other parts of the world. Intracerebral hemorrhage constitutes up to 30% of all strokes in Asian populations. In a Chinese study, a total of 551,163 people were supervised for 15 years; the annual average incidences of stroke and intracerebral hemorrhage were 236.6/100,000 and 131.0/100,000, respectively. The mean annual mortality rate of intracerebral hemorrhage was 78.3/100,000 and that of stroke in general was 124.5/100,000. The percentage of intracerebral hemorrhage among stroke cases was 55.4%. Among patients with intracerebral hemorrhage, 79.8% had hypertension, 30.6% had cardiovascular disease, 7.6% had diabetes mellitus, and 12.5% had an abnormally high level of lipid (170). The incidence of intracerebral hemorrhage in Japanese populations is also substantially high. In an epidemiological study from Japan, 350 patients with primary first-ever intracerebral hemorrhage who were treated during the 7-year period 1991 to 1998 were included. The crude and age- and sex-adjusted incidence rates for all types of intracerebral hemorrhage were 52 and 47 per 100,000 populations, respectively, for all ages. The most common site of intracerebral hemorrhage was the putamen, followed by the thalamus, lobar areas, brainstem, cerebellum, and caudate nucleus. The Glasgow Coma Scale scores on admission were best in patients with cerebellar hemorrhage and worst in those with brainstem hemorrhage (66).
In patients with acute stroke, bedside clinical evaluation does not always help in distinguishing cerebral infarction from intracranial hemorrhage. Accuracy of the Siriraj and Guy's hospital stroke scores is poor in distinguishing hemorrhagic from ischemic stroke (08). The “SCAN rule” may help in identifying intracerebral hemorrhage in patients with minor stroke. The presence of one or more of the following indicates intracerebral hemorrhage: systolic blood pressure 180 mmHg or more, diastolic blood pressure 110 mmHg or more, confusion, anticoagulation, or nausea and vomiting (94). Another study suggested that factors favoring ischemic strokes versus hemorrhagic strokes were diabetes, atrial fibrillation, previous myocardial infarction, previous stroke, and intermittent arterial claudication. Smoking and alcohol consumption favored hemorrhagic stroke (04). Patients with intracerebral hemorrhage require immediate neuroimaging for prompt diagnosis. Both computed tomography and magnetic resonance imaging are equally capable of identifying the presence of acute intracerebral hemorrhage (19).
Acute hematoma appears hyperdense on CT scan. The degree of hyperdensity depends on red blood cell concentration. Hematoma in patients with preexisting anemia may not be as hyperdense as that in patients with normal hematocrit. During the first 72 hours or longer, perihematomal edema produces a hypodense area around the lesion along with considerable mass effect. After this period, the central dense areas start becoming smaller, beginning at the periphery. The border of hematoma becomes irregular, and edema and mass effect are reduced. At this stage, some contrast enhancement may be seen at the periphery. Contrast study is not routinely needed unless underlying diseases such as infections or tumors are suspected. Certain hematoma characteristics may suggest specific mechanisms of hemorrhage. For example, fluid-blood level with relatively less perihematomal edema is characteristic of coagulopathy-associated hematoma. Careful attention should also be paid to the locations of hematoma. Underlying causes other than hypertension should be investigated when hematoma is not located in the common sites of hypertensive hemorrhage. Hematoma volume can be estimated by using the formula, Volume= A x B x C/2, with A, B, and C representing the maximum width, length, and thickness measured on the CT images (59). Hematoma volume is one of the strongest predictors of outcome.
Magnetic resonance imaging is of superior capability in identifying the extent of perihematomal edema and damage to the surrounding brain tissue by the compression effects. Hyperacute hematoma can be isointense and might elude detection by MRI. With the conversion of oxyhemoglobin to deoxyhemoglobin, hematoma might appear hypointense on T1-and T2-weighted images. In a matter of weeks, intracellular methemoglobin begins to accumulate and becomes hyperintense on T1-weighted images. Slow leakage of methemoglobin into the extracellular space follows and gives hematoma hyperintense appearance on T1- and T2-weighted images. Eventually, after complete reabsorption, hematoma becomes hypointense. When underlying tumors are suspected, MRI can provide more detailed imaging clues to the final diagnosis. MRI, however, offers limited additional diagnostic yield in basal ganglionic and thalamic hemorrhage (39). The accuracy of MRI relative to CT for the detection of hyperacute intracerebral hemorrhage had not been demonstrated in past. To address this issue, a prospective, multicenter study was performed at two stroke centers. Patients presenting with focal stroke symptoms within 6 hours of onset underwent brain MRI followed by non-contrast CT. Among 200 patients, for the diagnosis of any hemorrhage, MRI was positive in 71 patients with CT positive in 29 patients. For the diagnosis of acute hemorrhage, MRI and CT were used in an equal number of cases. Acute hemorrhage was diagnosed in 25 patients on both MRI and CT. In four other patients, acute hemorrhage was present on MRI but not on the corresponding CT--each of these four cases was interpreted as hemorrhagic transformation of an ischemic infarct. In three patients, regions interpreted as acute hemorrhage on CT were interpreted as chronic hemorrhage on MRI. In one patient, subarachnoid hemorrhage was diagnosed on CT but not on MRI. In 49 patients, chronic hemorrhage, most often microbleeds, was visualized on MRI but not on CT. It was concluded that MRI is as accurate as CT for the detection of acute hemorrhage (73). Newer neuroimaging techniques such as gradient-echo MRI have demonstrated the presence of cerebral microbleeds and the microangiopathy associated with hypertension and cerebral amyloid angiopathy. With the advent of gradient-echo MRI, patients with underlying cerebral amyloid angiopathy or hypertensive vasculopathy can be identified (148).
Angiography is not routinely done in patients with hypertensive bleeds. However, patients with lobar or primary intraventricular hemorrhage should always be subjected to angiography. Angiography should also be performed in patients who have subarachnoid blood associated with a parenchymal hematoma and in patients who have recurrent hemorrhages. The diagnosis of amyloid angiopathy is usually established by careful exclusion of other potential causes. Brain biopsy is not generally necessary.
The "spot sign" on CT angiography is usually present in several patients with acute primary intracerebral hemorrhage and has been reported to predict hematoma expansion (147). The “spot sign” is defined as spot-like or serpiginous foci of enhancement within the margin of a parenchymal hematoma without connection to outside vessels. The “spot sign” is usually greater than 1.5 mm in maximal dimension and has a Hounsfield unit density at least double that of background hematoma density. In a study, authors identified spot signs in 71 of 367 CT angiograms (19%), six of which were delayed spot signs (35). No patients with secondary intracerebral hemorrhage had a true CT angiography spot sign, but several spot sign mimics were identified, including micro-arteriovenous malformation, posterior communicating artery aneurysm, Moyamoya disease, and neoplasm-associated calcification (47). The island sign is another reliable computed tomography sign that reliably predicts hematoma expansion and poor outcomes in patients with intracerebral hemorrhage (86).
COVID-19-associated intracerebral hemorrhage has distinctive neuroimaging characteristics. Multifocal intracerebral hemorrhage is common in COVID-19-associated intracerebral hemorrhage. Noncontrast computed tomography features, such as intrahematomal hypodensity, heterogeneous density, blend sign, and irregular shape fluid level are also frequent among patients with COVID-19-associated intracerebral hemorrhage. In addition, frequent, ultra-early hematoma expansion was noted (116). In a multinational retrospective study, Mowla and colleagues in a logistic regression analysis noted that SARS-CoV-2, intracerebral hemorrhage occurred at younger age, and these patients also had higher median intracerebral hemorrhage scores, a higher incidence of diabetes mellitus, and lower platelet counts. In a univariate analysis, SARS-CoV-2 infection was significantly associated with higher in-hospital mortality (118).
In a Swedish study, out of 323 patients with intracerebral hemorrhage, 172 (53%) survived after 1 year, 127 (39%) survived after 5 years, and only 57 (18%) survived after 13 years (57). Poor prognostic factors for 30-day mortality include advanced age, low Glasgow coma score, infratentorial location, high intracerebral hemorrhage volume, and presence of intraventricular hemorrhage (59). Another study identified six independent admission criteria predicting the short-term functional outcome of intracerebral hemorrhage patients. These six significant and independent prognostic variables were decreased level of consciousness, severe hemiparesis, age older than 60 years, large hematoma size, midline shift, and intraventricular extension on CT (55). In one study, hydrocephalus score, ambient cistern effacement, intracerebral hemorrhage volume, and female sex were found to be independently associated with lower Glasgow Coma Score at presentation (179). In a study, the independent predictors of 28-day and 3-year mortality were male sex, high age, central and brain stem hemorrhage location, intraventricular hemorrhage, increased volume, and decreased consciousness level (181). Lack of early clinical improvement at 24 hours reliably predicts poor prognosis in intracerebral hemorrhage (175). In the INTERACT studies on intracerebral hemorrhage, the National Institutes of Health Stroke Scale (NIHSS) was a more effective predictor of 90-day outcomes than the Glasgow Coma Scale. A baseline NIHSS score of 10 or greater optimally predicted death or major disability, outperforming other scales. This finding was consistent across various subgroups and validated in the independent INTERACT1 database, highlighting NIHSS's utility in prognosticating outcomes for patients with mild-to-moderate intracerebral hemorrhage (176).
Patients with initial parenchymal hemorrhage volume of 60 cm3 or larger and Glasgow coma scale of 8 or less carry a 30-day mortality rate of 91%. Most patients with a volume of 30 cm3 or more remain severely disabled at 30 days (21). However, decreased level of consciousness at presentation in thalamic hemorrhage has a lower predictive value of long-term outcome when compared to hemorrhages in other locations (80). Pontine hemorrhage generally carries grave prognosis, with 55% mortality rate and 24% dependency-requiring disability (163). Prognosis for caudate hemorrhage is good. Most survivors from caudate hemorrhage remain independent.
An intracerebral hemorrhage scoring system consisting of admission Glasgow Coma Scale score, initial hematoma volume, presence of intraventricular hemorrhage, infratentorial intracerebral hemorrhage origin, and age has been found effective in predicting the prognosis (60).
Loan and colleagues assessed the prognostic significance of perihematomal edema volume on CT (91). The authors measured hematoma volume, perihematomal edema volume, and the sum of both measurements on cranial CT, which was performed at 3 or fewer days after symptom onset. Death or dependence at 1 year were primary outcome measures. The authors noted that hematoma volume and the combined volume of hematoma and perihematomal edema independently predicted a poor outcome. Perihematomal edema volume alone was not associated with a poor outcome at 1 year.
Both diabetes and admission hyperglycemia in nondiabetic patients have been observed as predictors of poor outcome after supratentorial intracerebral hemorrhage. This may be related to the greater incidence of cerebral and infectious complications in diabetic patients and of cerebral complications in hyperglycemic nondiabetic patients (122). Hyperglycemia, at admission, may independently increase the risk of early death in acute spontaneous intracerebral hemorrhage (74). Pathological HbA1c levels are significantly associated with and predisposing for deep intracerebral hemorrhage. Hematoma volumes are substantially increasing in patients with spontaneous lobar intracerebral hemorrhage who are older than 70 years (79). Hyponatremia is an independent predictor of in-hospital mortality in spontaneous intracerebral hemorrhage, and correction of hyponatremia, possibly, does not compensate its influence on mortality (78).
Important complications of intracerebral hemorrhage include hematoma expansion, perihematomal edema with increased intracranial pressure, intraventricular extension of hemorrhage with hydrocephalus, seizures, venous thrombotic events, hyperglycemia, increased blood pressure, fever, and infections. Prevention and early detection of these complications are important in the reduction of adverse effects early in the course of intracerebral hemorrhage and in the improvement of prognosis (11).
Perihematomal edema growth is a crucial prognostic factor in patients with intracerebral hemorrhage. An absolute growth in perihematomal edema volume (1.02 to 1.33 per 5 mL increase from baseline) was significantly associated with death or dependency at 90 days after adjustment for demographic, clinical, and hematoma parameter prognostic factors. Time from intracerebral hemorrhage onset to baseline computed tomography, baseline hematoma volume, 24-hour hematoma growth, and intraventricular extension were independent determinants of 24-hour perihematomal edema growth (169).
Fever is common after intracerebral hemorrhage. The term “central fever” is often used when no cause is identified. It correlates with intracerebral hemorrhage volume and third ventricular shift suggesting a role of hypothalamic compression in central fever. One study showed a trend towards a worse outcome with fever (36; 98).
Gastrointestinal bleeding is an important and sometimes serious complication in critically ill patients of intracerebral hemorrhage. On multivariate analysis, size of hematoma, septicemia, and low Glasgow coma score were identified as predictors of gastrointestinal hemorrhage (109). In anticoagulant-associated intracerebral hemorrhage, the mortality rate is greater than 50%, and most of the surviving patients remain disabled. In a study, age, Glasgow Coma Scale score, sepsis, and intracerebral hemorrhage volume were independent predictors of gastrointestinal bleeding (171).
Rebleeding in spontaneous intracerebral hemorrhage is a major cause of morbidity and mortality among stroke survivors. Multivariate logistic regression analysis indicated that hydrocephalus and serum interleukin-10 were independently associated with an increased probability of rebleeding (158).
Early seizures are a frequent complication in patients with spontaneous intracerebral hemorrhage; however, their occurrence does not influence outcomes. Early seizures were defined as seizures occurring within 7 days of stroke onset. The only factor associated with early seizures was cortical involvement of intracerebral hemorrhage (34).
IL-6 (interleukin 6), a proinflammatory cytokine, was found associated with severity and functional outcome after spontaneous intracerebral hemorrhage. Higher IL-6 blood levels were associated with larger initial hematoma volume, lobar location, larger perihematomal edema, and poorer outcome (modified Rankin Scale score) (81).
The presence of contrast extravasation on multidetector computed tomography angiography in patients with hyperacute-stage intracerebral hemorrhage is an independent and strong factor associated with poor outcomes. Any patient with intracerebral hemorrhage with such sign on multidetector computed tomography angiography should be monitored intensely and treated accordingly (85). In another study, out of the 131 patients, a spot sign was seen in 31 patients. In multivariate analysis, the spot sign predicted significant hematoma expansion, in-hospital mortality, and poor clinical outcome (133). Subarachnoid extension has also been found associated with poor prognosis, which is determined by a larger volume of the underlying intraparenchymal hematoma (28).
Hematoma enlargement. Hematoma expansion is an accurate predictor of poor outcomes from intracerebral hemorrhage (37). Several serial imaging studies have consistently demonstrated that enlargement of hematoma size due to active bleeding is a major cause of clinical deterioration within the first 3 hours after the onset of hemorrhage (23).
According to a published study, hematoma growth is common in acute intracerebral hemorrhage, any degree of growth being seen in 73% of patients (33). For each 10% increase in hematoma growth, there was a 5% increased hazard of death, a 16% greater likelihood of worsening by 1 point on the modified Rankin Scale, or 18% of moving from independence to assisted independence or from assisted independence to poor outcome on the Barthel index. The researchers observed that percentage hematoma growth increase, intraventricular hemorrhage, initial intracerebral hemorrhage volume, and clinical factors like low Glasgow Coma Score were all associated with increased mortality and poor functional outcome. A published analysis further confirms that most hematoma growth occurs early after onset of intracerebral hemorrhage (22). Larger hematomas on the baseline CT are associated with increased absolute intracerebral hemorrhage growth. A study observed that very small hematomas are unlikely to expand, by commonly used absolute growth definitions, and may represent a subgroup of patients with intracerebral hemorrhage destined towards good clinical outcomes (37). Treatment with recombinant activated factor VII limits hemorrhage growth in patients with spontaneous intracerebral hemorrhage. Contrast extravasation on CT angiography was found to be a significant predictor of subsequent hematoma expansion in patients presenting later than the first few hours after symptom onset (49). Presence of tiny, enhancing foci ("spot sign") within acute hematomas has been associated with hematoma expansion (157). Wada and colleagues prospectively studied 39 consecutive patients with spontaneous intracerebral hemorrhage by computed tomography angiography within 3 hours of symptom onset. Patients were dichotomized according to the presence or absence of the spot sign. Hematoma expansion occurred in 11 patients (28%) on follow-up. Seventy-seven percent of patients with and 4% without hematoma expansion demonstrated the spot sign. In a prospective cohort study, hematoma expansion occurred in 156 patients (19.1%) (24). In multivariable analysis, predictors of expansion were warfarin sodium use, the computed tomography angiography spot sign, and shorter time to computed tomography, as well as baseline hematoma volume. Hematoma expansion is more frequent in deep than lobar intracerebral hemorrhage (132).
Hematoma expansion occurs in children with intracerebral hemorrhage as well. In a study, hematoma expansion was noted in seven of 22 (32%) children (12). Three children had significant (greater than 33%) expansion; two required urgent hematoma evacuation. Hematoma expansion, in children, was not associated with poorer outcomes. The computed tomography angiography spot sign is associated with more intraoperative bleeding, more postoperative rebleeding, and larger residual intracerebral hemorrhage volumes in patients undergoing surgical hematoma evacuation for spontaneous intracerebral hemorrhage (25). Coagulation defects in children significantly enhance the risk of hematoma expansion (18).
Hyperglycemia is associated with greater hematoma expansion and poorer clinical outcomes after intracerebral hemorrhage. The effect of hyperglycemia on hematoma expansion and plasma kallikrein-mediated inhibition of platelet aggregation could be mimicked by infusing mannitol. These findings suggest that hyperglycemia augments cerebral hematoma expansion by plasma kallikrein-mediated osmotic-sensitive inhibition of hemostasis (90).
Oral anticoagulants are also associated with hematoma enlargement. The effect of warfarin on baseline intracerebral hemorrhage volume was studied in 183 consecutive cases of supratentorial intracerebral hemorrhage. Subjects were drawn from an ongoing prospective cohort study of intracerebral hemorrhage outcomes. The effect of warfarin on intracerebral hemorrhage expansion (increase in volume greater than or equal to 33% of baseline) was analyzed in 70 consecutive cases. Warfarin was found to be the only significant predictor of hematoma expansion. Expansion in warfarin patients was detected later in the hospital course compared with non-warfarin patients (44). Lower level of consciousness at presentation and larger initial intracerebral hemorrhage volume predict poor prognosis in patients with warfarin-associated intracerebral hemorrhage (185). Warfarin use was associated with larger initial intracerebral hemorrhage volume, but this effect was only observed for international normalized ratio values greater than 3.0. Larger intracerebral hemorrhage volume among warfarin users likely accounts for part of the excess mortality in this group (43). The presence of cerebral microbleeds may be an independent risk factor for warfarin-related intracerebral hemorrhage (154).
Regular aspirin use preceding the onset of intracerebral hemorrhage was associated significantly with hematoma enlargement during the first week after intracerebral hemorrhage. Authors observed poor short-term outcomes and increased mortality, probably attributable to rapid enlargement of hematomas, in subjects with intracerebral hemorrhage who had been taking regularly moderate doses of aspirin (median 250 mg) immediately before the onset of the stroke (135). The authors reviewed records of 251 consecutive patients hospitalized in their cerebrovascular center within 24 hours after the onset of intracerebral hemorrhage. Fifty-seven patients (23%) developed intracerebral hemorrhage during oral antiplatelet therapy. The major indication for antiplatelet therapy was the prevention of stroke recurrence. Antiplatelet therapy was predictive of an increase in the hematoma volume by more than 40% on the second hospital day and the need for emergent surgical evacuation of the hematoma (149). Higher mortality was found in antiplatelet-treated patients (44.9%) than in anticoagulated patients (31.1%). This may be related to a difference in the mean age of 78 versus 71 years (26). Previous use of antiplatelet agents was a predictor of hematoma enlargement in patients with intracerebral hemorrhage treated with rapid administration of antifibrinolytic agents and blood intracerebral hemorrhage pressure control. Treatment with HMG-CoA reductase inhibitors may also be a risk factor for increased intracerebral hemorrhage volume in spontaneous brain hemorrhages and could contribute to the hemorrhage’s volume progression (130). In people taking antiplatelet drugs platelet transfusion was found inferior to standard treatment in patients with intracerebral hemorrhage (09).
The presence of spot signs suggested an increased risk of hematoma expansion. Among the spot sign characteristics examined, the presence of three or more spot signs, a maximum axial spot sign dimension of 5 mm or more, and maximum attenuation of 180 Hounsfield units or more were independent predictors of significant hematoma expansion (35). Severe white matter hyperintensities have been found to be associated with larger intracerebral hemorrhage volumes and with hematoma expansion (92). Several other computed tomographic signs have been described that predict hematoma expansion and risk of death. These signs include black hole sign, swirl sign, heterogeneous density, blend sign, hypodensities within hematoma, irregular shape, and island sign (113; 114).
The FAST-MAG trial investigated prehospital magnesium sulfate treatment for acute stroke, including intracerebral hemorrhage (88). Analysis of 381 patients found that higher magnesium levels in the treatment group were linked to reduced hematoma expansion and neurologic deterioration, whereas no such association was seen in the placebo group. This suggests a potential hemostatic effect of magnesium in stroke treatment. However, the study's limitations call for further trials to confirm these findings.
Dementia risk. Moulin and coworkers noted that there is a substantial risk of incident dementia in dementia-free survivors of spontaneous intracerebral hemorrhage. It was thought that underlying cerebral amyloid angiopathy is an important factor contributing to the occurrence of new-onset dementia in these patients (117).
A meta-analysis (5435 eligible patients from 36 cohorts) found that four predictors (time from symptom onset to baseline imaging, intracerebral hemorrhage volume on baseline imaging, antiplatelet use, and anticoagulant use) were independent predictors of intracerebral hemorrhage growth. Spot sign on CT angiography had marginal predictive value (01).
Medical management. As per guidelines From the American Heart Association/American Stroke Association, management of patients with intracerebral hemorrhage should take place in an intensive care unit setting (19). Initial management of intracerebral hemorrhage is focused on maintaining breathing, circulation, and fluid and electrolyte balance. Patients who show a decreasing level of consciousness or signs of brainstem dysfunction require endotracheal intubation. Administration of intravenous fluids should be in the form of normal saline. Hypotonic solutions and intravenous glucose administration should be avoided. Fever has been known to aggravate brain injury; therefore, high body temperature needs to be aggressively treated by antipyretics such as acetaminophen or a cooling blanket. The primary causes of fever also need to be investigated and appropriately treated. Patients with acute primary intracerebral hemorrhage and hemiparesis/hemiplegia should have intermittent pneumatic compression for the prevention of venous thromboembolism (19). Seizures secondary to intracerebral hemorrhage can be seen within the first 24 hours, in which case an appropriate antiepileptic drug should always be used. A brief period of antiepileptic therapy, soon after intracerebral hemorrhage, has been suggested. The choice of antiepileptic drug should include a drug that can be given both orally and intravenously (19). Levetiracetam is a preferred antiepileptic drug in this setting (96). Levetiracetam has a neuroprotective effect against posthemorrhagic stroke brain injury (65). Syndrome of inappropriate antidiuretic hormone secretion and cerebral salt-wasting syndrome should be anticipated, and electrolyte disturbances should be corrected if present.
Increased intracranial pressure should be aggressively treated. Drainage of the cerebrospinal fluid with intracranial pressure monitoring is indicated in case of ventricular obstruction, which is commonly seen in hemorrhage in the caudate nucleus and thalamus. Intubation and hyperventilation is often the best way to acutely lower increased intracranial pressure. Hyperventilation to reduce PaCO2 to 30 mm Hg is used when herniation occurs from perihematomal edema. Mannitol is also used commonly to treat increased intracranial pressure. In a randomized evaluation, low-dose mannitol was not found beneficial in patients with intracerebral hemorrhage (110). In this study, 128 patients with CT-proven supratentorial intracerebral hemorrhage within 6 days of ictus were randomized into the study and control groups. The study group received mannitol 20%, 100 ml every 4 hours for 5 days, tapered in the next 2 days. The control group received sham infusion. Primary endpoint was 1-month mortality and secondary endpoint functional disability at 3 months assessed by Barthel index score. At 1 month, 16 patients died in each group. The primary and secondary endpoints were not significantly different between the two groups. Mannitol can cause renal failure. Fluid and electrolyte balance should be carefully monitored during mannitol therapy. Mannitol should not be routinely used because it may diffuse into the hematoma, aggravating edema and mass effect. Guidelines recommend that intracranial pressure should be monitored in patients who are in coma or show evidence of herniation, intraventricular hemorrhage, or hydrocephalus; the goal should be a cerebral perfusion pressure of 50 to 70 mm Hg. A ventricular drainage should be considered for patients with hydrocephalus and a depressed level of consciousness (61).
Corticosteroids, particularly dexamethasone, are commonly used for treatments in patients with subarachnoid hemorrhage and primary intracerebral hemorrhage despite the lack of evidence (41). Overall, there is no evidence of a beneficial or adverse effect of corticosteroids in patients with primary intracerebral hemorrhage.
Elevated blood pressure, observed in up to 56% of patients with intracerebral hemorrhage, may predispose to hematoma expansion. Cerebral blood flow is not substantially reduced in most patients with intracerebral hemorrhage. Lowering systolic blood pressure below 160 mm Hg in the first hours after intracerebral hemorrhage may prevent further bleeding. It was noted that aggressive antihypertensive therapy after intracerebral hemorrhage did not increase the rate of neurologic deterioration even when treatment was initiated within hours of symptom onset (75). To address this issue, a randomized controlled trial was conducted (03; 05). In this study, patients with acute spontaneous intracerebral hemorrhage along with elevated systolic blood pressure (150 to 220 mm Hg) were randomly assigned to early intensive lowering of blood pressure (target systolic blood pressure 140 mm Hg) or standard guideline-based management of blood pressure (target systolic blood pressure 180 mm Hg). The primary efficacy endpoint was a proportional change in hematoma volume at 24 h; secondary efficacy outcomes included other measurements of hematoma volume. Safety and clinical outcomes were assessed for up to 90 days. Mean proportional hematoma growth was 36.3% in the control group and 13.7% in the intensive group at 24 hours. The relative risk of hematoma growth was 36% lower in the intensive group than in the control group. The absolute risk reduction was 8%. In fact, early intensive blood pressure lowering treatment is clinically feasible, is well tolerated, and reduces hematoma growth (151). Labetalol, nitroprusside, hydralazine, and enalapril are preferred antihypertensives (Table 2). It was observed that intensive lowering of blood pressure (systolic level of less than 140 mm Hg within 1 hour) did not result in a significant reduction in death or severe disability in comparison to guideline-recommended treatment (systolic level of less than 180 mm Hg) with the use of the drug as per physician's choice. This study included 2794 participants; 1382 participants (52%) received intensive treatment, and 1412 participants received guideline-recommended treatment. Mortality was 11.9% in the group receiving intensive treatment and 12% in the group receiving guideline-recommended treatment (02). Intensive blood pressure lowering appears beneficial across a wide range of baseline systolic blood pressure levels. Target systolic blood pressure level of 130 to 139 mm Hg is likely to provide maximum benefit in acute intracerebral hemorrhage (06). A study further confirms the benefits of early intensive blood pressure lowering in patients with intracerebral hemorrhage receiving prior antithrombotic therapy. Prior antithrombotic therapy is associated with greater hematoma growth, and early blood pressure lowering leads to decreased hematoma volume (140). One study did not find any evidence to suggest that patients with intracerebral hemorrhage and spot sign benefit from intensive blood pressure reduction (115). A systematic review noted that a rapid reduction of systolic blood pressure by continuous intravenous administration of nicardipine during the initial 24 hours in patients with hyperacute intracerebral hemorrhage significantly lowered the risk of hematoma expansion and risk of 90-day mortality (150). In a multicenter trial across 10 countries, a care bundle protocol significantly improved outcomes in patients with acute intracerebral hemorrhage (95). This protocol focused on intensive early blood pressure lowering, strict glucose control, antipyrexia treatment, and rapid anticoagulation reversal. The study, involving 7036 patients, showed a lower likelihood of poor functional outcomes and fewer serious adverse events in the care bundle group compared to usual care. These findings advocate for incorporating this protocol in hospitals, highlighting its effectiveness in actively managing acute intracerebral hemorrhage, especially within hours of symptom onset.
|
• Labetalol 10 mg intravenous every 10 to 20 minutes, max 150 mg/day |
As hematoma growth in acute intracerebral hemorrhage is a dynamic process, treatment with early hemostatic therapy could lead to the prevention of early hematoma growth. The hemostatic properties of recombinant activated factor VII (rFVIIa) are established in patients with inherited or acquired hemophilia with inhibitors and in patients with congenital factor VII deficiencies. Its predominant action is limited to areas of injury, apparently without systemic activation of the coagulation cascade. Recombinant activated factor VII (rFVIIa) may be a valuable therapy during the hyperacute stage of intracerebral hemorrhage. A random evaluation demonstrated that treatment with rFVIIa within 4 hours after the onset of intracerebral hemorrhage limits the growth of the hematoma, reduces mortality, and improves functional outcomes at 90 days (101). Serious thromboembolic adverse events, mainly myocardial or cerebral infarction, occurred in 7% of rFVIIa-treated patients, as compared with 2% of those given a placebo. Another study by the same group of authors suggested that therapy with rFVIIa reduced hematoma growth but did not improve survival or functional outcome (102). In this study authors randomly assigned 841 patients with intracerebral hemorrhage to receive placebo, 20 micrograms of rFVIIa per kilogram of body weight, or 80 micrograms of rFVIIa per kilogram within 4 hours after the onset of intracerebral hemorrhage. The primary endpoint was poor outcome, defined as severe disability or death according to the modified Rankin scale 90 days after the stroke. Treatment with 80 micrograms of rFVIIa per kilogram resulted in a significant reduction in growth in volume of the hemorrhage. Despite this reduction in bleeding, there was no significant difference among the three groups in the proportion of patients with poor clinical outcomes (24% in the placebo group, 26% in the group receiving 20 micrograms of rFVIIa per kilogram, and 29% in the group receiving 80 microgram). Tranexamic acid, a potent antifibrinolytic drug, has been tried to prevent hematoma expansion in patients with intracerebral hemorrhage. In a systematic review with meta-analysis, Wang and colleagues noted that tranexamic acid reduced the risk of hematoma expansion in patients with intracerebral hemorrhage (160). However, in a meta-analysis of the data of 2800 patients from six trials, tranexamic acid had no effect on reducing the risk of 90-day mortality.
Warfarin-related intracerebral hemorrhage requires adequate transfusion of fresh frozen plasma (10 to 20 ml/kg) to achieve immediate normalization of prothrombin time along with vitamin K. For thrombolysis-related intracerebral hemorrhage, six units of cryoprecipitate (rich in fibrinogen) are generally recommended to increase the fibrinogen level to at least 150 mg/dl. Platelet concentrate may also be needed if a massive transfusion is necessary. Anticoagulation should be immediately reversed. Fresh frozen plasma is the standard treatment in most institutions. Prothrombin complex concentrate is an alternative, but issues of availability make its use impractical. The use of recombinant factor VIIa is a newer option (143). Early intervention focuses on a rapid correction of coagulopathy in order to prevent continued bleeding.
An updated Cochrane Review assessed the efficacy of hemostatic therapies in treating spontaneous intracerebral hemorrhage (40). Analyzing 20 randomized controlled trials with 4652 participants, it found that recombinant activated factor VII (rFVIIa) showed little effect on reducing death or dependence post-intracerebral hemorrhage. Antifibrinolytic drugs did not significantly affect death or dependence but slightly reduced intracerebral hemorrhage expansion. Platelet transfusion likely increased death or dependence after antiplatelet-associated intracerebral hemorrhage. The effectiveness of prothrombin complex concentrates versus fresh frozen plasma in anticoagulant-associated intracerebral hemorrhage was uncertain. Ongoing trials may enhance the certainty of these findings.
Surgical management. Data from both experimental and clinical studies suggest that early surgical evacuation of supratentorial cerebral and parenchymal intracerebral hemorrhage may be beneficial. However, a number of randomized studies and several meta-analyses have not been able to provide a clear indication for surgery in intracerebral hemorrhage. It has been suggested that hematoma evacuation should be offered to a subset of patients with moderate-sized hemorrhages and associated moderate neurologic deficits, specifically those patients who are likely to survive the primary bleed but with significant permanent neurologic deficits. Minimal-access surgical techniques may offer advantages over standard large craniotomies, although a role for stereotactic aspirations has not yet been established. The timing of any surgery may also be important with theoretical advantages associated with early and thorough clot evacuation (99). However, guidelines for the management of spontaneous intracerebral hemorrhage did not find any evidence to suggest that ultra-early (within 7 hours of symptom onset) craniotomy improved the functional outcome or mortality (19). On the contrary, a study observed that patients with intracerebral hemorrhage who undergo craniotomy or craniectomy have high morbidity and mortality. The most common complications reported were pulmonary, renal, and thromboembolic (123).
In a prospective randomized Surgical Trial in Intracerebral Hemorrhage (STICH) trial authors compared early surgery with initial conservative treatment for patients with intracerebral hemorrhage, including 1033 patients from 83 centers in 27 countries (107). Patients were randomized to early surgery (503) or initial conservative treatment (530). At 6 months, 51 patients were lost to follow-up, and 17 were alive with unknown status. Of 468 patients randomized to early surgery, 122 (26%) had a favorable outcome compared with 118 (24%) of 496 randomized to initial conservative treatment. It was observed that patients with spontaneous supratentorial intracerebral hemorrhage in neurosurgical units show no overall benefit from early surgery when compared with initial conservative treatment. A prospective randomized study of craniotomy and early hematoma removal versus best medical management was performed in 108 patients with primary intracerebral hemorrhage. Surgical or medical treatment was initiated within 8 hours post ictus. Analysis of outcome revealed a significantly higher percentage of Glasgow coma scale scores higher than 3 for the surgical patients, compared with those of the conservative group (33% and 9%, respectively). However, the mortality rates between operated and conservatively managed patients did not differ significantly (121). However, in a 2008 meta-analysis (including 10 eligible trials with 2059 participants), authors concluded that in patients with primary supratentorial intracerebral hemorrhage, surgery was associated with statistically significant reduction in the odds of being dead or dependent compared with medical management alone (126). Authors also thought that the result was not very robust. Endoscopic hematoma evacuation provided the quick, adequate decompression of intracerebral hemorrhage. It has been demonstrated that the outcome with endoscopic hematoma evacuation was better than the CT-guided hematoma removal (120). Emergency surgery in deteriorating patients with large anticoagulation-associated intracerebral hemorrhage has been found beneficial despite the presence of clinical and radiological signs of herniation before the evacuation (127). Patients with intracerebral hemorrhage complicated by intraventricular extension and hydrocephalus often require an external ventricular drain. Ventricular drainage should be considered in all stuporous or comatose patients with intraventricular hemorrhage and acute hydrocephalus. Given the lack of benefit seen in a STICH trial, emergency surgical evacuation within 72 hours of onset should be reserved for patients with large lobar hemorrhages, substantial mass effect, and rapidly deteriorating clinical condition. A meta-analysis indicated that there was improved outcome with surgery if it was undertaken within 8 hours of hemorrhage, or the volume of the hematoma was 20 to 50 mL, or the Glasgow Coma Score was between 9 and 12, or the patient was aged between 50 and 69 years (51). In addition, there was some evidence that more superficial hematomas with no intraventricular hemorrhage might also benefit. Stereotactically guided clot evacuation have been shown to be safe and effective in improving the outcomes (183).
Guidelines for the management of spontaneous intracerebral hemorrhage suggested that surgical evacuation of hematoma is necessary in cerebellar hemorrhage, especially if the hematoma is larger than 3 cm in diameter and the patient is deteriorating neurologically or if the hematoma is associated with a fourth ventricle obstruction causing increased intracranial pressure or hydrocephalus or showing evidence of compression over brainstem (111; 112; 103; 19). A current published meta-analysis that included four observational studies (data of 6580 patients from 64 hospitals in the United States and Germany) revealed that surgical hematoma evacuation, in comparison to conservative treatment, was not associated with improved functional outcome at 3 months (77).
The STICH II trial suggested that early surgery might have a small survival benefit for patients with superficial lobar intracerebral hemorrhage. In this study, 307 of 601 patients were randomly assigned to early surgery and 294 to initial conservative treatment. All patients were followed up at 6 months. Finally, 297 and 286 were included in the analysis, respectively. It was observed that 174 (59%) of patients in the early surgery group had an unfavorable outcome versus 178 (62%) patients in the initial conservative treatment group (absolute difference 3•7%) (108).
Limited data exist about the benefits of intraventricular hemorrhage removal in humans. There are several theoretical benefits of intraventricular clot removal, including limitation of inflammation, edema, and cell death as well as restoration of cerebral spinal fluid flow, intracranial pressure homeostasis, improved consciousness, and shortening of intensive care unit stay (56).
Prevention of recurrent intracerebral hemorrhages. Treatment of hypertension is the most effective way to prevent recurrent hemorrhages in patients with primary intracerebral hemorrhage. Avoidance of other risk factors like smoking, alcohol, and cocaine further helps in reducing the risk of recurrent hemorrhages (19).
Rehabilitation and recovery measures. “2022 Guideline for the Management of Patients With Spontaneous Intracerebral Hemorrhage” recommended early rehabilitation and recovery measures in the management of intracerebral hemorrhage are crucial for better quality of life. These guidelines favor early supported discharge for mild to moderate intracerebral hemorrhage and coordinated multidisciplinary inpatient team care. The guidelines further recommend implementation of rehabilitation activities, such as stretching and functional task training 24 to 48 hours after moderate intracerebral hemorrhage (50). A trial from China showed that commencing rehabilitation within 48 hours of intracerebral hemorrhage improves survival and functional outcomes at 6 months after stroke (89).
All contributors' financial relationships have been reviewed and mitigated to ensure that this and every other article is free from commercial bias.

Ravindra Kumar Garg DM
Dr. Garg of King George's Medical University in Lucknow, India, has no relevant financial relationships to disclose.
See Profile
Steven R Levine MD
Dr. Levine of the SUNY Health Science Center at Brooklyn has no relevant financial relationships to disclose.
See ProfileNearly 3,000 illustrations, including video clips of neurologic disorders.
Every article is reviewed by our esteemed Editorial Board for accuracy and currency.
Full spectrum of neurology in 1,200 comprehensive articles.
Listen to MedLink on the go with Audio versions of each article.
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Stroke & Vascular Disorders
Dec. 29, 2024
Stroke & Vascular Disorders
Oct. 29, 2024
Stroke & Vascular Disorders
Oct. 29, 2024
Stroke & Vascular Disorders
Oct. 29, 2024
Stroke & Vascular Disorders
Oct. 29, 2024
Stroke & Vascular Disorders
Oct. 29, 2024
Sleep Disorders
Oct. 14, 2024
Developmental Malformations
Sep. 22, 2024