Infectious Disorders
Creutzfeldt-Jakob disease
Dec. 27, 2024
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Worddefinition
At vero eos et accusamus et iusto odio dignissimos ducimus qui blanditiis praesentium voluptatum deleniti atque corrupti quos dolores et quas.
Rabies is an acute viral infection of the nervous system that has both encephalitic and paralytic clinical forms. Transmission of the rabies virus usually occurs in saliva from the bite of a rabid animal. Although rabies is an ancient disease, human rabies continues to be a problem in developing countries, particularly in Asia and Africa. In this article, the author reports that bat rabies virus variants cause most cases of human rabies in the United States, and there is often no history of a bat bite or even contact with bats. Worldwide, dogs are the most important vector. Although rabies can be prevented after an exposure with wound cleansing and the administration of the rabies vaccine and of rabies immune globulin, there is no established effective therapy once rabies develops; the disease is almost always fatal. In developed countries, rabies is often not recognized until late in the course of the disease or postmortem because physicians are not familiar with the clinical manifestations.
|
• Bats are the vector for most human rabies cases in North America whereas dogs are the most important vector worldwide. | |
|
• Rabies can be effectively prevented after a recognized exposure, but the disease almost invariably is fatal once clinical disease develops. | |
|
• Rabies should usually be suspected from the clinical features even if there is no history of an animal exposure. | |
|
• Laboratory tests may confirm a diagnosis of rabies, but negative tests do not exclude rabies, and the tests may need to be repeated. | |
|
• There is no effective therapy for rabies; more research is needed to gain a better understanding of the mechanisms involved in the disease and for the development of novel therapies. |
Rabies has been present since antiquity, and perhaps the earliest reference was in the pre-Mosaic Eshnunna Code of Mesopotamia in about 2300 BC (71). The works of Hippocrates, Aristotle, and Celsus referred to rabies in humans and animals (25; 06; 41). Ancient Chinese writings also indicate that rabid dogs were recognized centuries before the birth of Christ (97). Dog rabies was a serious problem in the Old World during the 19th century, and it was also present in colonial North America (78). In the early 19th century, Zinke demonstrated experimentally that the infective agent causing rabies was transmitted in saliva by painting saliva from a rabid dog into incisions in healthy animals (104). Louis Pasteur developed the first rabies vaccine, and Joseph Meister, a boy who had been bitten by a rabid dog, was the first patient immunized against rabies in 1885. Rabies remains a serious public health problem in both humans and dogs in developing countries (101).
|
• Rabies usually begins with nonspecific prodromal symptoms that develop 1 to 3 months after an exposure. | |
|
• Human rabies has both encephalitic and paralytic forms of disease. | |
|
• Hydrophobia is a characteristic feature of encephalitic rabies. |
Clinical features of human rabies have been reviewed (42). Rabies usually develops 1 to 3 months (or, rarely, a few days or more than a year) after exposure, which is most often from an animal bite. Prodromal symptoms, including fever, chills, malaise, fatigue, insomnia, anorexia, headache, anxiety, and irritability, may last for a few days. About one half of patients develop pain, paresthesias, or pruritus at or close to the bite site, which likely reflects infection and associated inflammation in local dorsal root ganglia or cranial sensory ganglia (34).
Encephalitic rabies. About 80% of patients with rabies develop an encephalitic (also called furious) form of rabies and 20% develop a paralytic form. In the encephalitic form, patients have episodes of generalized arousal or hyperexcitability, which are separated by lucid periods (89). They may have aggressive behavior, confusion, and hallucinations. Fever is common, and signs of autonomic dysfunction include hypersalivation, piloerection (gooseflesh), sweating, and priapism. Nuchal rigidity and seizures may occur. About one half of patients develop hydrophobia, a characteristic manifestation of rabies. Patients may initially experience pain in the throat or have difficulty swallowing. On attempts to swallow, they experience contractions of the diaphragm and other inspiratory muscles, which last for about 5 to 15 seconds. Subsequently, the sight, sound, or even mention of water (or of any liquids) may trigger the spasms. A draft of air on the skin may have the same effect (aerophobia). The disease may progress through paralysis, coma, and multiple organ failure, and eventually it causes death. Increased libido and hypersexuality may be early manifestations of rabies in females (42), and spontaneous ejaculation occurs rarely in males (02).
Paralytic rabies. In paralytic rabies, flaccid muscle weakness develops early in the course of the disease, often beginning in the bitten extremity and spreading to the other extremities and facial muscles. Sphincter involvement, pain, and sensory disturbances also occur. Hydrophobia is unusual, although bulbar and respiratory muscles eventually become involved. Patients with paralytic rabies usually survive longer than those with the encephalitic form of the disease. Encephalopathy, hydrophobia, and aerophobia are more common in dog-acquired rabies, whereas abnormal cranial nerve, motor and sensory examinations, tremor, myoclonus, local sensory symptoms, symptoms at the exposure site, and local symptoms in the absence of a bite or scratch are more common in bat-acquired cases (84).
Rabies is virtually always fatal, despite attempts at aggressive therapy. Most survivors have received one or more doses of rabies vaccine prior to the onset of clinical manifestations of disease.
Survival from rabies has been well documented in about 33 cases and 31 of these 33 were immunized with at least one dose of rabies vaccine before the onset of clinical disease (44; Jackson 2021). Patients with rabies who are managed aggressively in critical care units often develop multiple organ failure and have cardiac arrhythmias, a variety of respiratory complications, diabetes insipidus, and inappropriate secretion of antidiuretic hormone (34; 30). Death usually occurs within 14 days of the onset of clinical manifestations. A 17-year-old patient survived but did not have typical clinical features, did not require intensive care, and did not develop anti-rabies virus neutralizing antibodies (13); indeed, the patient probably did not actually have rabies. A similar young patient from California with rapid recovery was also reported (14).
A 13-year-old boy, who was a resident of a rural community in Mexico, was bitten on his foot by a dog when riding a horse. He received a complete course of rabies vaccination with suckling mouse brain vaccine beginning on the day after the exposure. Rabies immune globulin was not given. Thirteen days later, he developed leg and lumbar pain, headache, fever, vomiting, dysphagia, and pharyngeal spasms. A corneal touch preparation for immunofluorescent detection of rabies virus antigen was inconclusive. The boy was admitted to a hospital with periods of excitability, photophobia, nystagmus, tremor, hypertonicity, and increased deep tendon reflexes. He died six days after the onset of his clinical illness.
An autopsy showed mononuclear inflammatory infiltrates in the brain and spinal cord, but no viral inclusion bodies (Negri bodies). Immunostaining demonstrated rabies virus antigen in the hippocampus, medulla, spinal cord, and rare Purkinje cells (56).
Rabies is an acute infection of the central nervous system caused by infection by rabies virus and very rarely by other lyssaviruses.
|
• During most of the incubation period, rabies virus is located close to the site of entry. | |
|
• Rabies virus spreads within axons of peripheral nerves and axons in the CNS by fast axonal transport. | |
|
• Rabies virus spreads from the CNS to multiple organs along neural pathways. |
Rabies virus spreads from the animal's infected brain to the salivary glands, and the virus may be present in the saliva. The virus is transmitted by a bite or, rarely, by contamination of skin lesions or mucous membranes by saliva. The steps in rabies pathogenesis are illustrated and have been reviewed (43). The virus is probably localized close to the site of entry in the host during most of the incubation period. Rabies virus can bind to the nicotinic acetylcholine receptor at neuromuscular junctions. The virus spreads to the CNS (centripetal spread) in the axoplasm of peripheral nerves by retrograde fast axonal transport (43) and infects local peripheral sensory ganglia and spinal cord neurons. The neural cell adhesion molecule has also been identified as another rabies virus receptor (81). Rabies virus disseminates rapidly within the CNS by fast axonal transport, and widespread infection of neurons with selectivity occurs. Subsequently, the virus spreads along neural pathways to multiple organs (centrifugal spread), including the salivary glands, adrenal medulla, heart, and skin (50). Salivary gland infection is essential for transmission in rabies vectors.
The basis of the neuronal dysfunction in rabies is unknown (43). Experimental studies in mice after peripheral inoculation have shown that structural abnormalities in neuronal processes may explain the fatal clinical disease in rabies (74), which may be due to oxidative stress (47) related, at least in part, to mitochondrial dysfunction due to interaction of the rabies virus phosphoprotein with mitochondrial complex I (52; 51). There is also evidence of mitochondrial dysfunction in naturally infected dog and human brains (33). Distinct pathologic features of paralytic rabies have not been defined. Hydrophobia is likely due to selective infection of neurons that inhibit brainstem neurons near nucleus ambiguus, resulting in exaggeration of defense reflexes that normally protect the respiratory tract.
Rabies results from infection of the CNS by rabies virus, which is usually transmitted by the saliva of a biting animal. Transmission has also occurred by inhalation of aerosolized rabies virus (in laboratory accidents and probably also in the Frio cave in Texas, which is inhabited by millions of Brazilian free-tailed bats). Eight cases of rabies have occurred due to corneal transplantation (42). Transmission occurred from donors via organ (or vascular segment) transplantation in Texas (four cases) (79), Florida (one case) (86), Germany (three cases) (60), Kuwait (four cases) (22; 73), China (seven cases), and Syria (two cases) (103; 16; 31; 15; 57; 73).
In a bite exposure, the animal's teeth breach the skin; in a nonbite exposure, saliva (or CNS tissue) contaminates an open wound, scratch, abrasion, or mucous membrane. Aerosolized rabies virus is inhaled (enters the brain by an olfactory route), or rabies virus-infected tissues (eg, cornea) or organs (eg, kidney) are transplanted.
|
• Worldwide, dogs are the most important vectors of rabies. | |
|
• In North America, wild animals, especially bats, are the most important rabies vectors. |
During the period 2000 to 2021, an average of 2.5 human deaths per year (median 2; range 0 to 8) occurred in the United States from rabies (36). In 2021, there were five human cases of rabies in the United States, and none in each of 2019, 2020, and 2022 (58; 59).
Rabies vectors include dogs, cats, bats, skunks, foxes, and raccoons. Canine rabies, endemic in many countries of Asia and Africa, is responsible for the majority of human cases worldwide. Over 20,000 human deaths are estimated annually in India, and almost all are a result of dog bites (101). Other domestic animals, such as cats, cattle, and other farm animals are less important vectors. In 1996, a woman died in Australia from a newly identified lyssavirus, Australian bat lyssavirus, closely related to rabies virus, which was likely transmitted from an insectivorous bat. A second Australian case occurred in 1998, which was associated with another bat (flying fox) virus variant (32). A third case occurred in an 8-year-old child in 2013 (27). In 2002, a man died in Scotland due to a European bat lyssavirus type 2, which was transmitted by a Daubenton bat (Myotis daubentonii) (26; 66). In 2006, a patient in South Africa died from Duvenhage virus infection, which was transmitted from a bat (69). In 2007, a patient died in The Netherlands from Duvenhage virus infection, which was transmitted from a bat in Kenya (85).
In the United States and Canada, wild animals, including bats, foxes, skunks, and raccoons (bats and foxes in western Europe), are the main threat for transmission to humans because control of rabies in domestic animals has been successful in these countries (87; 59).
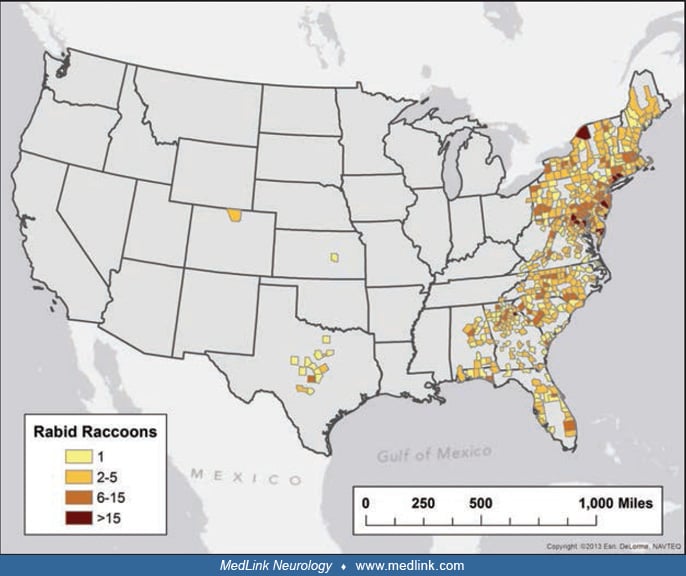
Transmission from bats is most common, and bat-related rabies viruses have been identified in the majority of human cases diagnosed in the United States since 1980 (67; 20; 65). The silver-haired bat rabies virus variant is often identified in human victims, and bites from these small bats may not be recognized (46). There has been a rabies epizootic in raccoons in the mid-Atlantic and northeastern states that spread into Ontario in 1999 (88), and raccoons are currently present throughout the eastern United States.
The first recognized human case caused by a raccoon rabies virus variant occurred in 2003 (12). In 2004, four recipients died with rabies transmission from organ transplants and from a vascular segment transplant (for a liver) performed in Texas (09; 79). In 2005, three organ recipients died after transmission from another donor in Germany (60). A kidney recipient died of rabies in Florida in 2013, and the donor had been infected by the raccoon rabies virus variant (86). Four patients died of rabies after organ transplantation performed from a donor in Kuwait (22; 73), seven patients died of rabies after transplantations from three different donors in China, and two patients died after transplantations in Syria (103; 16; 31; 15; 73). Over 15,000 individuals in the United States receive rabies postexposure prophylaxis every year (63). The public health response to a single rabid kitten in New Hampshire, including postexposure prophylaxis of 665 persons, cost about 1.5 million U.S. dollars (10). Nonbite exposures rarely cause rabies but may warrant postexposure prophylaxis under certain circumstances (03; 63). Since 1950, only a minority (38%) of cases of rabies in the United States and Canada have had a clear history of an animal bite exposure (40). Human-to-human transmission of rabies is not well documented except by tissue and organ transplantation (24). The cost of rabies control in the United States alone exceeds 300 million U.S. dollars per year.
Over the past 30 years there has been much progress in the control of dog-mediated rabies in Latin America and the Caribbean (28). However, much work remains to be done in Asia and Africa in order to reduce the burden of rabies transmitted by dogs, which are currently responsible for 60% and 36% of global burden of human deaths, respectively (101).
|
• The risk of developing rabies from an animal exposure needs assessment. | |
|
• Only healthy dogs, cats, and ferrets can be observed after an exposure prior to initiating postexposure rabies prophylaxis. | |
|
• Postexposure prophylaxis includes wound cleansing and administration of rabies vaccine (usually four doses) and rabies immune globulin, including infiltration of the wounds. |
Rabies is preventable with appropriate management after an exposure. Current recommendations on rabies prevention were updated in 2008 and are summarized in the Morbidity and Mortality Weekly Report (63). Even minor deviations from recommendations have resulted in rabies.
After a human is bitten by a dog, cat, or ferret, the animal should be captured, confined, and observed for a period of at least 10 days and examined by a veterinarian prior to its release. If the animal is a stray or unwanted, or if signs of rabies are present or develop during the observation period, the animal should be killed immediately and the head transported under refrigeration for a laboratory examination. The brain should be examined for the presence of rabies virus, usually via an antigen detection method using the fluorescent antibody technique and viral isolation using cell culture or mouse inoculation. The incubation period for animals other than dogs, cats, and ferrets is uncertain; hence, they should be killed immediately after an exposure and the head submitted for examination. In high-risk exposures and in areas where canine rabies is endemic, rabies prophylaxis should be initiated before the results are obtained from the laboratory examination (35). If the result is negative, one may safely conclude that the animal's saliva did not contain rabies virus and, if immunization has been initiated, it should be discontinued. If an animal escapes after an exposure, it should be considered rabid unless information from public health officials indicates that this is unlikely, and rabies prophylaxis should be initiated. Current recommendations indicate that the physical presence of a bat may warrant postexposure prophylaxis when a person (such as a small child or sleeping adult) is unable to reliably report contact that could have resulted in a bite (63). However, in light of the low risks and high costs, recommendations for bedroom exposures to a bat while sleeping and without known physical contact have been questioned (21).
Postexposure prophylaxis includes local wound care and both active and passive immunization (38; 63). Local wound care should be given as soon as possible after all exposures, even if immunization is delayed, pending the results of an observation period. All bite wounds and scratches should be washed thoroughly with soap and water. Devitalized tissues should be debrided. Tetanus prophylaxis and measures to control bacterial infection should also be given when warranted.
Human diploid cell vaccine is an inactivated (with beta-propiolactone) vaccine grown in a human diploid cell line for active immunization against rabies virus; a purified chick embryo cell culture vaccine was licensed in the United States in 1997 (63). Other vaccines grown in either primary cell lines (hamster or dog kidney) or continuous cell lines (Vero cells) are also satisfactory and are available in other countries (101). A regimen of four 1-mL doses of rabies vaccine (reduced from five doses) (72) should be given intramuscularly in the deltoid area (anterolateral aspect of the thigh is also acceptable in children). Ideally, the first dose should be given as soon as possible after the exposure, but failing that, it should be given regardless of the length of a delay. Three additional doses should be given on days 3, 7, and 14 after the first dose. Immunocompromised individuals should receive an additional dose of vaccine and subsequent additional doses if rabies serology demonstrates inadequate titers (72). Pregnancy is not a contraindication for immunization (17; 63). Live vaccines should not be given for one month after rabies immunization. Local reactions (pain, erythema, edema, and pruritus) and mild systemic reactions (fever, myalgias, headache, and nausea) are common. Antiinflammatory medications and antipyretics may be used, but immunization should not be discontinued. Systemic allergic reactions are uncommon and anaphylactic reactions may be treated with epinephrine and antihistamines. Corticosteroids may interfere with the development of active immunity. Immunosuppressive medications should not be administered during postexposure therapy unless they are essential. The risk of developing rabies should be carefully considered before deciding to discontinue vaccination because of an adverse reaction. A serum neutralizing antibody determination is necessary only after immunization of immunocompromised patients. Less expensive vaccines, derived from neural tissues, have been used in developing countries; however, these vaccines are associated with a high rate of serious neuroparalytic complications. Worldwide, over 10 million people receive postexposure vaccination against rabies each year.
Human rabies immune globulin should also be administered as passive immunization for protection before the development of immunity from the vaccine. It should be given at the same time as the first dose of vaccine and no later than seven days after the first dose. Rabies vaccine and human rabies immune globulin should never be administered at the same site or in the same syringe. The recommended dose of human rabies immune globulin is 20 IU/kg; larger doses should not be given because this may suppress active immunity from the vaccine. After wounds are washed, they should be infiltrated with human rabies immune globulin (if anatomically feasible), and the remainder of the dose should be given intramuscularly (eg, in the gluteal area). If the exposure involves a mucous membrane, the entire dose should be administered intramuscularly. In 2018, the World Health Organization indicated that under certain circumstances the remainder of the dose does not need to be administered after local infiltration of the wound(s). With multiple or large wounds, the human rabies immune globulin may need to be diluted for adequate infiltration of all of the wounds. Adverse effects of human rabies immune globulin include local pain and low-grade fever. If human rabies immune globulin is unavailable, purified equine rabies immune globulin can be used in the same manner at a dose of 40 IU/kg. The incidence of anaphylactic reactions and serum sickness has been low with equine rabies immune globulin products (95). Worldwide, inadequacies in postexposure prophylaxis of animal bite victims have failed to prevent most human deaths from rabies (96).
Pre-exposure rabies prophylaxis should be considered for people at risk of rabies exposures (including certain travelers). Two doses of rabies vaccine (recently reduced from three doses) may be given intramuscularly on days 0 and 7 for pre-exposure vaccination, and rabies antibody titers should be checked every 6 to 24 months, depending on the level of risk (70). When an exposure occurs in a pre-immunized individual, two booster doses of vaccine should be administered without human rabies immune globulin.
Other viral encephalitides may show behavioral disturbances with fluctuations in the level of consciousness. However, hydrophobic spasms are not observed, and it is unusual for a conscious patient to have brainstem signs in other encephalitides. Herpes simiae (B virus) encephalomyelitis (which is transmitted by monkey bites) is often associated with a shorter incubation period than that of rabies, and recovery may occur (94). Cases of rabies have been misdiagnosed as Creutzfeldt-Jakob disease (11). In malaria-endemic areas of Africa, fatal cases of rabies were misdiagnosed as malaria in 12% of cases (61).
Tetanus also has a shorter incubation period (usually 3 days to 21 days) than rabies, and unlike rabies, it is characterized by sustained muscle rigidity involving paraspinal, abdominal, masseter (trismus), laryngeal, and respiratory muscles with superimposed brief recurrent muscle spasms (08). In patients with tetanus, consciousness is preserved, no CSF pleocytosis is present, and the prognosis is much better than for those with rabies.
Autoimmune encephalitis is an important consideration (01), particularly anti-N-methyl-D-aspartate (anti-NMDA) receptor encephalitis. Anti-NMDA receptor encephalitis occurs in young patients (especially females) and is characterized by behavioral changes, autonomic instability, hypoventilation, and seizures; it has been recognized that this autoimmune disease rivals viral etiologies as a cause of encephalitis (29). Postvaccinal encephalomyelitis is the most important differential diagnosis in patients immunized with a vaccine derived from neural tissues (eg, Semple vaccine). Local symptoms at the site of the bite, hydrophobic spasms, and alternating intervals of agitation and lucidity are not seen in postvaccinal reactions (35).
The diagnosis of rabies may be difficult if an exposure is not suspected. Patients and their relatives may not be able to recall an animal exposure even when questioned directly. There may be a history of recent travel in an endemic area. Rabies may present with bizarre neuropsychiatric symptoms, and it is most commonly misdiagnosed as a psychiatric or laryngopharyngeal disorder.
Rabies hysteria (a somatic symptom) likely occurs as a psychological response to the fear of rabies (100), and it may be the most difficult differential diagnosis. It is characterized by a shorter incubation period than rabies, aggressive behavior, inability of the patient to communicate, and a long course with recovery.
Paralytic rabies resembles Guillain-Barré syndrome, and pathologically the conditions may also be similar (76). A report from India showed that three of 83 (3.4%) pediatric patients initially treated for Guillain-Barré syndrome actually had paralytic rabies (75). Local symptoms at the site of the bite, piloerection, early or persistent bladder dysfunction, and fever are more suggestive of paralytic rabies. Guillain-Barré syndrome may occasionally occur as a postvaccinal complication from rabies vaccines derived from neural tissues, particularly the suckling mouse brain vaccine (82).
|
• Imaging features are not specific for rabies diagnosis. | |
|
• Key tests for rabies diagnosis include detection of rabies virus antigen or RNA in saliva, skin biopsies, CSF, or brain tissues. | |
|
• Rabies virus may be isolated using culture techniques. | |
|
• In rabies, neutralizing anti-rabies virus antibodies may be detected in the CSF or, in unimmunized patients, in sera. |
Routine hematologic and biochemical studies are not useful in rabies diagnosis. The electroencephalogram may show nonspecific abnormalities. CT scans of the head are usually normal. MR imaging may show increased T2 and fluid attenuated inversion recovery (FLAIR) signal intensity in gray matter areas of the brain parenchyma, including the basal ganglia, thalami, hypothalami, brainstem, and frontal and parietal lobes (80). The lesions are usually symmetrical and predominantly involve deep gray matter structures, but symmetrical involvement of white matter in frontal and parietal regions may also be seen (80). There may also be involvement of nerve roots, plexuses, and peripheral nerves (54; 07). Lesions may enhance after administration of contrast (55). Diffusion restriction may be observed on diffusion-weighted images (07). Characteristic imaging differences between encephalitic and paralytic rabies cases have not been recognized (54; 07).
CSF analysis is often abnormal. Anderson and colleagues found a CSF pleocytosis in 59% of cases during the first week of illness and in 87% after the first week (04). Mononuclear cells predominate in the CSF, the protein level may be mildly elevated, and the glucose level is usually normal. Serum neutralizing antibodies against rabies virus are not usually present in unimmunized patients until after the 10th day of illness (34), and death may occur before they develop. Early in the illness, rabies virus may be occasionally isolated from the saliva or CSF (04). A skin biopsy may confirm a diagnosis of rabies during life (90). Rabies virus antigen may be detected in skin biopsies using the fluorescent antibody technique. Antigen may be demonstrated in small nerves of skin taken from the nape of the neck, which is rich in hair follicles. Detection of antigen in corneal impression smears is less sensitive than in skin biopsies (90). Diagnosis of rabies using brain biopsies has not been adequately assessed. Postmortem CNS tissues can be assessed for rabies virus antigen and viral isolation. Postmortem brain tissue and CSF for confirming a rabies diagnosis can be collected via needle biopsy and needle aspiration, respectively, in a minimally invasive autopsy (23).
Detection of rabies virus RNA has been demonstrated from brain tissues, saliva, and CSF using polymerase chain amplification (67; 83). One study showed a higher specificity (100%) and sensitivity (98% or greater) of RT–PCR (heminested protocol) on skin biopsy specimens than on saliva specimens, although saliva is much easier to collect, and sensitivity on saliva was 100% when three successive samples were analyzed (18). Negative tests for rabies virus antigen or RNA do not exclude rabies unless performed on brain tissues. Repeat specimens may need to be collected and tested to confirm a diagnosis or rabies.
|
• Critical care is essential for aggressive management of rabies cases. | |
|
• No effective specific agents have known efficacy for the therapy of rabies. | |
|
• The “Milwaukee Protocol” should not be used. | |
|
• Palliative measures are important for the majority of patients who develop rabies. |
Antiviral therapy and a variety of immunotherapies, including ribavirin, favipiravir, and interferon-alpha, have been unsuccessful in the treatment of rabies (64; 92; 05; 98). Therapy is supportive, and survival has been prolonged with intensive care. Therapeutic options have been reviewed, especially for the situation in which an aggressive approach is desired (38; 45). The case of a rabies survivor in Wisconsin has demonstrated that aggressive care can be successful, even when rabies vaccine has not been given before the onset of clinical disease (99). However, the importance of the specific therapy used to treat this patient is now doubtful (39; 44; 102), and similar approaches in at least 62 patients plus six additional patients who died after receiving therapy with favipiravir have failed (98; 44). It is highly doubtful that therapeutic coma has any efficacy for therapy of rabies, and this approach to therapy (dubbed the "Milwaukee Protocol") lacks a clear scientific rationale and should now be discontinued (102; 44). Studies with ketamine in a mouse model of rabies were disappointing (93), and therapy with minocycline actually aggravated the disease (48). Administration of corticosteroids is also not recommended (38). Therapeutic trials in naturally infected dogs may facilitate advances in identifying effective new therapies (53). A palliative approach may be appropriate for many patients with rabies, and the use of specific agents has been discussed in reviews (91; 44; 45). As a result of the possibility of transmission, health care workers should employ body substance precautions and wear gowns, gloves, face shields, or both particulate respirators and goggles and may require postexposure prophylaxis after high-risk contact with a patient with rabies. Failure to consider a diagnosis of rabies and initiate appropriate precautions may lead to recommendation of postexposure rabies prophylaxis to a large number of healthcare workers, eg, 440 individuals after postmortem diagnosis in British Columbia (68).
Except for rare patients who received one or more doses of rabies vaccine prior to the onset of clinical disease, rabies is virtually always fatal despite attempts at aggressive therapy. To date, there have been 33 well-documented survivors of rabies, including 17 cases from India since 2014 (19; 62; 44).
Healthy infants have been born to mothers with rabies (37), although a single case report has documented transplacental transmission (77). Transplacental transmission of rabies virus has been reported in animals both naturally and experimentally (03). Pregnancy is not a contraindication to postexposure prophylaxis (17; 63).
No special considerations exist for anesthesia other than body substance precautions.
All contributors' financial relationships have been reviewed and mitigated to ensure that this and every other article is free from commercial bias.

Alan C Jackson MD
Dr. Jackson of Lake of the University of Calgary has no relevant financial relationships to disclose.
See Profile
John E Greenlee MD
Dr. Greenlee of the University of Utah School of Medicine has no relevant financial relationships to disclose.
See ProfileNearly 3,000 illustrations, including video clips of neurologic disorders.
Every article is reviewed by our esteemed Editorial Board for accuracy and currency.
Full spectrum of neurology in 1,200 comprehensive articles.
Listen to MedLink on the go with Audio versions of each article.
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Infectious Disorders
Dec. 27, 2024
Infectious Disorders
Dec. 12, 2024
Infectious Disorders
Dec. 10, 2024
Peripheral Neuropathies
Nov. 16, 2024
Infectious Disorders
Nov. 15, 2024
Infectious Disorders
Nov. 12, 2024
Infectious Disorders
Nov. 12, 2024
Infectious Disorders
Oct. 08, 2024