General Neurology
Use of focused ultrasound in neurologic disorders
Jan. 13, 2025
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Worddefinition
At vero eos et accusamus et iusto odio dignissimos ducimus qui blanditiis praesentium voluptatum deleniti atque corrupti quos dolores et quas.
Patients on chronic dialysis or those who suffer from chronic renal failure are prone to develop a variety of neurologic complications. Encephalopathy is most likely and is caused by multiple metabolic derangements, but the precise pathophysiology still remains unknown. Reversible posterior leukoencephalopathy syndrome or posterior reversible encephalopathy syndrome are common CNS disorders in patients with uremia. It is characterized by typical radiologic findings in the posterior regions of the cerebral hemisphere and needs special attention because of its potential reversibility on prompt treatment. Dialysis itself is associated with several types of encephalopathies, including disequilibrium syndrome, subdural hematoma, and, rarely, Wernicke encephalopathy. Dialysis dementia, associated with excessive aluminum in dialysates, is now rarely encountered. Antibiotic-associated encephalopathy has been described in chronic kidney disease. It generally presents with delirium and is associated with poor prognosis. The lentiform fork sign may be a reliable neuroimaging sign for an early diagnosis of uremic encephalopathy. Encephalopathy is a common neurologic complication in hospitalized COVID-19 patients. In a large number of these patients, renal failure is a dominant underlying pathogenic mechanism that is responsible for cerebral dysfunction. Unrecognized renal insufficiency is common among patients with acute stroke and is associated with adverse short-term outcomes. Neuropathy generally only develops at glomerular filtration rates of less than 12 milliliter/min. Carpal tunnel syndrome is more common in hemodialysis patients than in the general population. Pruritus is a common complication of end-stage renal disease, affecting about one third of dialysis patients. For patients with autonomic neuropathy, specific treatments, including sildenafil for impotence and midodrine for intradialytic hypotension, are effective. A high prevalence of sleep apnea in hemodialysis patients has been noted. In this article, the author reviews the clinical features, pathogenesis, and management of central and peripheral nervous system complications encountered in patients with renal failure.
|
• Chronic renal failure causes a variety of neurologic disorders affecting the central nervous system and the peripheral nervous system. | |
|
• These complications include diffuse encephalopathy, seizures, stroke, movement disorders, sleep alterations, polyneuropathy, mononeuropathies, and myopathy. | |
|
• Dialysis is associated with several types of encephalopathies, including posterior reversible encephalopathy syndrome, disequilibrium syndrome, subdural hematoma, and Wernicke encephalopathy. | |
|
• Several mechanisms involved include toxic metabolic accumulation, hyperkalemia, hypercoagulability, immunologic disturbances, and acid base disequilibrium. | |
|
• Early diagnosis of these complications is important for their prevention and for proper management. |
Uremia occurs when renal failure results in the systemic retention of nitrogenous and other waste products. Such metabolic derangements may cause dysfunction of various tissue types including those of the central and peripheral nervous systems (58). Uremic encephalopathy is diagnosed when renal failure is responsible for alteration of mental status (18). In 1962, Kennedy and associates identified dialysis disequilibrium syndrome as a characteristic symptom complex of mental confusion, headache, and muscle twitching following rapid correction of uremia by hemodialysis in patients with chronic renal failure (31). Arieff developed an animal model of the condition by exposing acutely uremic dogs to rapid hemodialysis (05). For the first time, in 1972, Alfrey described dialysis dementia in five chronically dialyzed patients who developed intermittent speech abnormalities followed by a distinct encephalopathy that was later believed to be a result of aluminum toxicity to the CNS (02). In 1971, Dyck and colleagues described uremic neuropathy in detail based on their extensive nerve conduction studies in these patients. Warren and Otieno, in 1975, reported carpal tunnel syndrome in patients on intermittent hemodialysis. In 1954, the team of John Merrill, Hartwell Harrison, and David Hume, headed by Joseph Murray, ushered in the modern era of human organ transplantation.
The diagnosis of uremic encephalopathy may be straightforward in a patient with renal compromise, a glomerular filtration rate less than 10% of normal, and altered mentation. Mild renal insufficiency should not be regarded as sufficient to cause encephalopathy, and other causes must be sought. There is no clear-cut direct correlation between the blood urea nitrogen level and the degree of encephalopathy as rapidity of rise of blood urea nitrogen and comorbidities unique to the individual patient may influence clinical severity. In a patient with advanced renal disease and alteration of mental status, several other clinical conditions should be considered in the differential diagnosis. The clinical features of uremic encephalopathy are nonspecific and do not allow differentiation from the other causes of metabolic encephalopathy. Hypocalcemia, hyperphosphatemia, hyperkalemia, metabolic acidosis, and hepatic insufficiency may all be present in a patient with renal failure and may independently result in encephalopathy, requiring specific management. Patients with both hepatic and renal failure are particularly prone to encephalopathy, as the diseased kidney may not be able to adequately clear accumulated plasma ammonia. In severe liver disease, the serum urea and creatinine may not adequately reflect renal function. Diseases affecting the kidney frequently involve other tissues, including those of the CNS, resulting in encephalopathic symptoms that are independent of the consequences of renal failure. For example, systemic lupus erythematosus may result in renal failure that is insufficiently severe to cause encephalopathy but may also give rise to a fulminating CNS vasculitis. Renal failure is often associated with compromise of the immune system, rendering such patients liable to infection, including that of the brain or meninges. Altered mentation from such an infective source may be amenable to treatment if recognized and not attributed to underlying renal dysfunction. Subdural hematoma is a frequently missed cause of encephalopathy and has been reported in 1% to 3.3% of patients undergoing hemodialysis. Drugs that are excreted or significantly metabolized by the kidneys may accumulate in renal insufficiency and result in a toxic encephalopathy. In this case, dialysis may also clear the drug from the blood and will not serve to distinguish this cause of encephalopathy. Cephalosporins can cause nonconvulsive status epilepticus in patients with renal failure, and the clinical picture may resemble uremic encephalopathy (51; 21).
Encephalopathy. Uremic encephalopathy generally develops in patients with acute or chronic renal failure when the glomerular filtration rate declines below 10% of normal (58). The clinical features are variable, tend to occur early, are not clearly distinguishable from those of other metabolic encephalopathies, but may respond to hemodialysis (18). Features of uremic encephalopathy are characteristic of a metabolic encephalopathy and may include sluggishness, easy fatigability, daytime drowsiness and insomnia, itchiness, inability to sustain attention or to perform complex cognitive tasks, slurred speech, anorexia, nausea and vomiting, restlessness, poor memory, diminished sexual interest, emotional lability, confusion, convulsions, stupor, and preterminal coma (45; 08). The coexistence of features suggestive of depression of neural activity together with those of neural excitation (ie, twitching, agitation, myoclonus, asterixis, seizures) is somewhat distinctive to uremic encephalopathy (58). In general, uremic encephalopathy is more severe and fulminating in patients with acute rather than chronic renal failure. Symptoms are promptly relieved following restoration of renal function, as occurs with hemodialysis or after successful renal transplantation (09). The seizures are usually generalized but may also be focal in nature, particularly where there is underlying brain pathology. Seizures are more common in acute (40%) than in chronic renal failure (10%). Asterixis refers to the sudden loss of muscle tone that classically results in a “flapping tremor” but is not specific to uremia.
Uremic myoclonus is often multifocal and involves the extremities. Uremic myoclonus in humans resembles the reticular reflex form of postanoxic action myoclonus. Research in cats suggests that this phenomenon arises in the brainstem medullary reticular formation. Nonepileptic focal and multifocal myoclonus has been seen in a few uremic patients using gabapentin and, in some patients, using the related anticonvulsant, pregabalin (72). Anatomical involvement of basal ganglia may cause chorea.
Antibiotic-associated encephalopathy has been described in end-stage kidney disease. Antibiotic-associated encephalopathy generally presents with delirium. Patients with chronic kidney disease with antibiotic-associated encephalopathy are associated with poor prognosis. Wang and Liu assessed the risk factors of antibiotic-associated encephalopathy in a cohort of 102 patients with chronic kidney disease along with 120 with chronic kidney disease without encephalopathy as controls (67). Coronary heart disease and decreased levels of hemoglobin, albumin, uric acid, and blood phosphorus predicted antibiotic-associated encephalopathy in patients with chronic kidney disease. Huang and colleagues observed antibiotic-associated encephalopathy in 4.4% of a cohort of 2104 patients with end-stage kidney disease (28). Anuria, history of central nervous system disorder, and hypoalbuminemia predicted antibiotic-associated encephalopathy. Ceftriaxone use in patients with chronic kidney disease at a dosage in excess of 2 gm/day for over 14 days enhanced the risk of developing encephalopathy (68).
Sagliker syndrome is a syndrome that has been described in patients with chronic renal failure and severe and late secondary hyperparathyroidism who suffered from severe skull and facial bone changes. Sagliker syndrome is characterized by disfiguring human face appearances and neuropsychiatric disorders. The most frequent neurologic manifestations are headache, polyneuropathy, cranial neuropathy, fatigue, and psychiatric disorders (24).
Dialysis itself is associated with several types of encephalopathies including the disequilibrium syndrome, subdural hematoma, and rarely, Wernicke encephalopathy. The symptoms of dialysis disequilibrium syndrome typically follow the initial treatment with hemodialysis of a patient with end-stage renal failure. Dialysis disequilibrium syndrome in a patient who has been repeatedly dialyzed is unusual. Symptoms may manifest at any time from the start of dialysis to 8 hours after its completion (34). Patients with extremely high levels of blood urea nitrogen (above 175 mg/dL) are at a higher risk of experiencing dialysis disequilibrium syndrome (49). Symptoms may be relatively mild (nausea, vomiting, fatigue, headache, blurred vision, muscle cramps and twitching, restlessness, anorexia) but may also include cardiac arrhythmias, disorientation, hypertension, tremor, seizures, and syncope (13). Dialysis disequilibrium syndrome is usually self-limited, with recovery several hours after completion of dialysis but occasionally taking up to several days. Hemodialysis patients are at risk for thiamine deficiency because of low intake and accelerated loss of thiamine during dialysis. The patients with thiamine deficiency may present with the clinical triad of Wernicke encephalopathy (ophthalmoplegia, ataxia, and altered consciousness), and intravenous administration of thiamine leads to its complete disappearance (29).
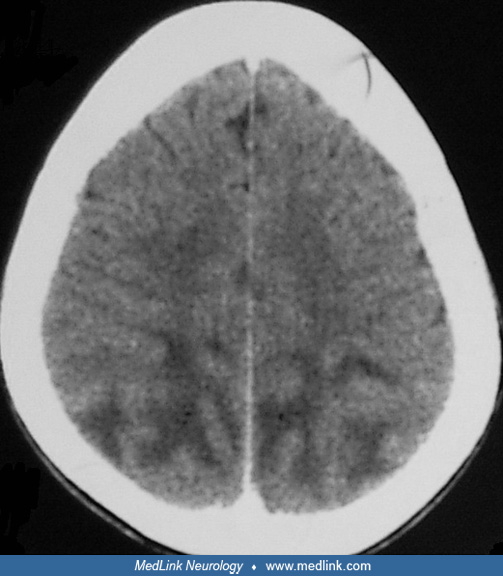
Posterior reversible leukoencephalopathy syndrome is a frequently encountered disorder in patients with uremia. This syndrome is characterized with typical radiologic findings in the posterior regions of the cerebral hemisphere and cerebellum. The symptoms include headache, nausea, vomiting, visual disturbances, focal neurologic deficits, and seizures. A variety of different etiologies have been reported in patients with renal failure such as hypertension, drug (erythropoietin, cyclosporine, or tacrolimus) neurotoxicity, uremia, and fluid and electrolyte disturbance. With early diagnosis and prompt treatment, the syndrome is usually fully reversible (23; 60; 25).
Predominant brainstem or cerebellar edema is rare in hypertensive encephalopathy and usually affects patients with secondary hypertension. Despite the severity of the radiologic findings, clinical features of brainstem involvement are uncommon. It is important to diagnose this entity as soon as possible because the symptoms and brainstem lesions are reversible following treatment and because it is important to exclude brainstem ischemia as an alternate diagnosis (10; 54; 22). Posterior reversible encephalopathy syndrome could be the first presentation of chronic kidney disease (16).
In series of 11 pediatric patients, authors graded neurologic symptoms as (1) mild (headache, nausea or vomiting, or tremor), (2) moderate (vision change), and (3) severe (mental dysfunction, cerebellar symptoms, seizures, recurrence of seizures, and coma). Magnetic resonance imaging features were graded as (1) subtle change, (2) abnormal large areas, and (3) complete involvement of the regions. The common symptoms were seizures, headache, nausea or vomiting, coma, and vision change. All but one patient developed hypertension, and seven patients had received calcineurin inhibitors. Symptoms were more severe in girls (69).
Hyponatremia and its rapid correction in patients with renal failure receiving hemodialysis may cause osmotic demyelination syndrome with damage to the pontine and extrapontine areas of the brain. Kim and coworkers described extrapontine lesions in addition to the pontine lesions in a patient with hemodialysis-associated osmotic demyelination syndrome due to end-stage renal disease. The patient exhibited lesions on bilateral middle cerebellar peduncles. He presented with progressive gait disturbance and postural instability. Accompanying symptoms included peduncular hallucinations and mild cognitive dysfunction (37). Severe hyponatremia with renal impairment is common in COVID-19 patients, and it predicts increased risk of encephalopathy and in-hospital mortality (20).
A unique syndrome of acute bilateral basal ganglia lesions, which presents with parkinsonism, altered mental status, dysarthria, and dysphagia in association with specific imaging findings in the basal ganglia, is an uncommon syndrome seen almost exclusively in patients with diabetes mellitus and renal failure (70; 71). In a cohort of 70 patients with uremia, authors noted that 15 patients had basal ganglia lesions on prospective follow up. Six of these patients also demonstrated a movement disorder (parkinsonism and dystonia). The majority of patients with basal ganglionic lesions were females, had diabetes and higher frequencies of abnormal renal dysfunction, metabolic derangements, and white matter hyperintensities in brain (26).
Galloway-Mowat syndrome is an inherited condition that manifests with severe encephalopathy, featuring microcephaly, developmental delay, and early-onset intractable epilepsy. Patients also typically show renal involvement from the onset (57).
Chronic kidney disease (defined as a reduced glomerular filtration rate or albuminuria) is considered a strong risk factor for stroke. A meta-analysis revealed that baseline estimated glomerular filtration rate less than 60 ml/min/1.73 m2 was independently related to incident stroke (46). Another meta-analysis revealed that microalbuminuria was strongly and independently associated with stroke risk (47). Chronic kidney disease was also predictive of poor outcomes after stroke (65). Pereg and coworkers noted that unrecognized renal insufficiency is common among patients with acute stroke and is associated with adverse short-term outcomes (56). These authors noted that of the 7900 patients with stroke, 5571 (70.5%) had normal renal function, 1510 (19.1%) had recognized renal insufficiency, and 819 (10.4%) had unrecognized renal insufficiency. Mortality rates were higher in patients with recognized and unrecognized renal insufficiency. Patients with intracerebral hemorrhage who have preexisting renal failure have a worse prognosis and higher rates of in-hospital mortality compared to those with normal renal function (33). Deteriorating renal function is considered a risk factor for developing dementia (38).
Neuropathy. The peripheral neuropathy is of insidious onset, progressing over months, and has been estimated to be present in 60% to 100% of patients on dialysis. Neuropathy generally only develops at glomerular filtration rates of less than 12 ml/min (41). Pathologically, it is characterized by axonal degeneration with secondary segmental demyelination. There is a marked predilection for large-diameter neurons. Unmyelinated and small myelinated neurons are relatively spared. The most prevalent symptoms early in the course of uremic neuropathy are distal paresthesias, sometimes with pruritus. The earliest signs are loss of vibration sense in lower limbs and impairment of ankle reflexes. A more severe form of neuropathy is characterized by ascending sensory and motor dysfunction, although this is infrequent. Uremic neuropathy may also be subclinical and detectable only by electrophysiological studies. A partly reversible acute uremic neuropathy, which is probably caused by the metabolic disturbances of end-stage renal failure, may mimic Guillain-Barré syndrome or chronic inflammatory demyelinating polyneuropathy. Autonomic dysfunction is another important component of uremic neuropathy. Impairment of the parasympathetic system is more frequent than sympathetic damage. A defective regulation of the heart rate, due mostly to an afferent lesion, is more common than damage of reflex blood pressure control. Both sympathetic and parasympathetic autonomic dysfunctions are reversible at a relatively early stage with renal transplantation. Carpal tunnel syndrome is more common in hemodialysis patients than in the general population. Arteriovenous fistula has also been identified as a possible cause of carpal tunnel syndrome. Rare but serious ischemic monomelic neuropathy is another type of mononeuropathy resulting from placement of forearm arteriovenous fistulas. This condition is a medical emergency; immediate surgical closure of the fistula is required to avoid severe and permanent neurologic dysfunction. Cranial nerve involvement in uremia is rare. Several cases of ischemic optic neuropathy have been reported. The pathogenesis of anterior ischemic optic neuropathy primarily involves interference with the posterior ciliary artery blood supply to the optic nerve. Uremic patients often have coexisting pathology such as hypotension, hypertension, atherosclerosis, or anemia, which may predispose them to anterior ischemic optic neuropathy. The immediate institution of dialysis, corticosteroid therapy, correction of anemia, and immediate management of hypotension can restore vision loss in some of these patients (07). Hearing loss is a common finding in patients with end-stage renal failure. Uremic toxins, ototoxins, and axonal nerve damage appear to be likely pathogenic factors.
Myopathy. Patients with chronic uremia develop a nonspecific proximal myopathy characterized by muscle weakness and reduced exercise capacity. The pathogenesis of proximal muscle weakness in uremia is uncertain but may be related to secondary hyperparathyroidism or osteodystrophy. Often it is associated with osteomalacic myopathy. Possible contributing mechanisms include altered vitamin D metabolism, carnitine deficiency, and insulin resistance. A study of patients with uremia and uremic rats demonstrated that muscle weakness and its electrophysiological correlates may be rapidly induced by uremic solutes and rapidly reversed when the solutes are removed by dialysis. Another observation of this study was that fast-twitch muscles are more readily affected by uremic conditions than slow-twitch muscles (27). The myopathy seen in renal failure is often associated with bone pain and tenderness. Acute myopathies in patients with renal failure are caused by water and electrolyte disturbances. If muscle weakness occurs episodically, periodic paralysis secondary to hypokalemia should be suspected. A systematic review suggested that oxidative stress can be responsible for some aspects of uremic myopathy (30). Oxidative stress can lead muscle cells into a catabolic state, and chronic oxidative stress leads to wasting.
Aouizerate and coworkers described a unique case of renal failure-associated calciphylaxis, also termed calcific uremic arteriolopathy (04). This is a life-threatening condition usually observed in patients with end-stage renal disease on chronic dialysis or after renal transplantation. Patients present with rapidly progressive unexplained systemic vasculopathy, muscle atrophy, and proximal weakness. Quadriceps muscle biopsy reveals diffuse vascular calcific deposits on medium- and small-sized vessels (04).
Uremia has been shown to accelerate sarcopenia, the loss of muscle mass and strength that results from the process of aging. Sarcopenia in uremic patients has multifactorial etiology including hormonal, immunologic, and myocellular changes, metabolic acidosis, reduced protein intake, and physical inactivity. Patients with chronic uremia also show some other pathophysiological evidence of aging, like immune dysfunction, telomere attrition, and the presence of low-grade inflammation (39; 14).
Sleep disturbances. Sleep disorders are common in dialysis patients. Insomnia is reported in almost 70% of the patients who are on dialysis. Excessive daytime sleepiness is also often reported by these patients. Direct effects of uremic encephalopathy and of somnogenic cytokines have been suggested as the cause of excessive daytime sleepiness in addition to the sleep disturbances that increase daytime sleepiness by impairing nocturnal sleep efficiency (53). A high prevalence of sleep apnea in hemodialysis patients has been noted. The dominant type of sleep apnea is obstructive sleep apnea. Some patients may have central sleep apnea. Uremia, metabolic acidosis, and body mass index are predictors of obstructive sleep apnea whereas PaO2, PaCO2, and cardiothoracic ratio are predictors of central sleep apnea (63). Other sleep disturbances, such as nightmares and narcolepsy, are less frequently encountered.
Restless legs syndrome. Restless legs syndrome is a common cause of sleep disturbance and is frequently experienced by patients on hemodialysis (59). Restless legs syndrome occurs in 40% of uremic patients. The underlying etiology of restless legs syndrome in patients with uremia is not clear. Various factors, including anemia and iron deficiency, have been proposed to play a major role.
Uremic pruritus. Pruritus is a common complication of end-stage renal disease, affecting about one third of dialysis patients. Severe pruritus not only affects quality of life but is also associated with poor outcome in chronic hemodialysis patients. Findings suggest that the neurophysiology of itch is similar to that of pain. Possibly, the two phenomena also closely interact in end-stage renal disease patients, who often also experience uremic neuropathy (50). A review on the subject noted that uremic pruritus is possibly either caused by toxins, peripheral neuropathy, immune alterations, or endogenous opioid dysregulation. In many controlled trials, drugs that act on opioid dysregulation mechanisms (like nalfurafine) have been found effective in relieving pruritis (66).
Among patients with end-stage renal disease, nervous system dysfunction remains a major cause of disability. The encephalopathy may initially worsen with periods of dialysis and almost certainly relates to altered metabolic states in association with ionic changes and possibly impaired synaptic function. The long-term prognosis of such patients rests on the availability of treatment and the nature of the underlying renal pathology.
Chronic renal failure of any etiology has the potential to cause uremic encephalopathy and other neurologic complications when the glomerular filtration rate falls below approximately 10% of normal. Acute renal failure is characterized by a rapid decline in the glomerular filtration rate, often a consequence of acute medication toxicities precipitating acute interstitial nephritis and acute tubular necrosis. Oliguria and extracellular fluid accumulation produce edema, hypertension, and congestive heart failure. Associated electrolyte abnormalities include hyperkalemia, hyponatremia, acidosis, and secondary hyperthyroidism. All of these primary and secondary effects of acute renal failure contribute to the development of uremic complications. Aggressive, rapid hemodialysis is associated with the development of dialysis disequilibrium syndrome. Risk factors for severity include malignant hypertension, severe uremia, and preexisting intracranial pathology predisposing to cerebral edema (17). The principal factor that results in dialysis dementia is chronic aluminum intoxication. It is considered the most severe manifestation of aluminum toxicity in conjunction with renal disease. Aluminum intoxication is rare in subjects with normal renal function, as aluminum is effectively cleared by the kidneys. Current cases of dialysis dementia have occurred only in dialysis units with greater than 20 µg/L aluminum concentration in the water supply. Etiologies of uremic neuropathy and uremic myopathy are not yet well understood. Toxic effects of a dialyzable toxin, vitamin B and other nutritional deficiencies, elevated levels of parathyroid hormone, and enzyme inhibition by uremic toxins are some of the proposed hypotheses for pathogenesis of neuropathy. Abnormal calcium metabolism may be responsible for uremic myopathy.
The precise biological basis of encephalopathy and other neurologic complications in patients with renal failure is largely unknown. It is likely that multiple factors are involved in the causation of these complications (45). Studies in the past suggested that the levels of urea and creatinine themselves did not correlate directly with encephalopathy, but retention of uremic toxins remains an important cause of uremic encephalopathy. However, approximately half of the osmotically active elements in the uremic brain have not been definitively identified. The remainders are termed “idiogenic osmoles” and may play an undetermined role. The fact that dialysis clears uremic encephalopathy suggests that the molecules responsible are water soluble and small to moderate in size. However, an autopsy study of patients with renal diseases, as compared to controls, showed a significant cerebral increase of urea, phenylethanolamine, and gamma-aminobutyric acid (61). Several authors have postulated that chronic renal failure-associated atherosclerosis and endothelial dysfunction result from accumulation of certain “uremic factors.” These factors include a variety of guanidino compounds, which have been shown to be nitric oxide synthase modulators both in vitro and in vivo (11). One report suggests that plasma levels of uremic toxins (eg, 3-carboxy-4-methyl-5-propyl-2-furanpropionate, hippurate, and indoleacetate) are closely correlated with the degree of uremic encephalopathy, suggesting that uremic toxins are involved in uremic encephalopathy. Uremia can cause inhibition of brain-to-blood transport, leading to accumulation of endogenous metabolites and drugs in the brain (12). An increase in total calcium content of the cerebral cortex accompanied by increased levels of cytosolic calcium in synaptosomes are common findings in rats with renal failure. These synaptosomes respond inappropriately to depolarization, which can impair neurotransmitter metabolism. Brain gamma-aminobutyric acid content, norepinephrine, and acetylcholine release uptake and degradation are affected by uremia. This may explain certain neurologic dysfunctions in uremia (62).
Renal dysfunction also appears to predispose the brain to posterior leukoencephalopathy, possibly because of increases in systemic blood pressure, metabolic abnormalities, or fluid overload (23). Disequilibrium syndrome is a complication of rapid hemodialysis. Brain edema is presumably caused by the “reverse urea effect.” Rapid dialysis leads to a urea gradient between blood and brain cells. Hemodialysis removes urea more slowly from the brain than from the plasma. Because of a higher concentration of urea in brain cells in comparison to blood, water flows into the brain causing brain edema (09). Alternatively, it has been suggested that after hemodialysis, patients have transient paradoxical metabolic acidosis within the central nervous system, displacing Na(+) and K(+) from organic anions and making them osmotically active, leading to cerebral edema (49). Renal failure causes oxidative stress. Oxidative stress is a potential mediator of uremic encephalopathy as well. An experiment indicated that acute kidney injury leads to oxidative stress in the brain, especially in the hippocampus and in the frontal cortex (40).
The mechanism of uremic neuropathy is also not precisely known. The observation that neuropathy improves with dialysis suggests that accumulation of dialyzable toxic substances is responsible for nerve loss. It had been postulated that neurotoxic compounds deplete energy supplies in the axon by inhibiting nerve fiber enzymes required for maintenance of energy production. Inhibition of the metabolism of B vitamins and concomitant nutritional deficiencies might be responsible for neuropathy. Myoinositol, a precursor of phosphoinositide, was found to be elevated in patients with chronic renal failure and had been held responsible for neuropathy. This substance is poorly eliminated by hemodialysis but efficiently excreted by transplanted kidneys. However, subsequent experimental studies did not support this hypothesis. Even elevated levels of parathyroid hormone had been considered neurotoxic. Calcium metabolic disorders, like increased parathyroid hormone levels, vitamin D deficiency, and impaired calcium transport, have been considered responsible for uremic myopathy (58). Studies utilizing axonal excitability techniques have suggested a hypothesis for the pathophysiology of uremic neuropathy. Nerves of uremic patients have been shown to exist in a chronically depolarized state prior to dialysis with subsequent improvement and normalization of resting membrane potential after dialysis. The degree of depolarization correlates with serum potassium, suggesting that chronic hyperkalemic depolarization plays an important role in the development of nerve dysfunction in end-stage kidney disease (41).
Acute kidney injury is common in the intensive care unit. It is often associated with nervous system involvement. The pathogenesis of acute uremic encephalopathy in patients with acute kidney injury is poorly understood. Experimental studies have noted inflammatory and functional changes in brain cells. Mice with acute kidney injury (compared with sham mice) had increased neuronal pyknosis and microgliosis in the brain. Acute kidney injury also led to increased levels of the proinflammatory chemokines keratinocyte-derived chemoattractant and granulocyte-colony stimulating factor in the cerebral cortex and hippocampus and increased expression of glial fibrillary acidic protein in astrocytes in the cortex and corpus callosum. In addition, extravasation of Evans blue dye into the brain suggested that the blood-brain barrier was disrupted (48).
Approximately 5% of patients suffering from COVID-19 have severe form of disease, which is characterized by acute respiratory distress syndrome, septic shock, and multiorgan dysfunction. Approximately 25% of hospitalized patients of COVID-19 develop acute kidney injury. Kidneys are damaged because of direct viral invasion and injury caused by dysregulated of the immune responses (cytokine storm) (01). Encephalopathy is a common neurologic complication in hospitalized patients of COVID-19. In large number of these patients, renal failure is a dominant underlying pathogenic mechanism that is responsible for cerebral dysfunction (19; 03).
The incidence of neurologic complications of renal failure in a given population parallels that of severe renal failure of all causes.
Issues of primary prevention and risk of development of neurologic complications of renal failure relate directly to the many possible causes of kidney injury. In a patient with established and advanced renal disease, renal transplantation and maintenance dialysis serve to prevent or postpone uremic encephalopathy.
Nephrogenic systemic fibrosis is a rarely encountered condition seen in patients with advanced kidney disease with or without dialysis. Nephrogenic systemic fibrosis is characterized by thickening and darkening of a large area of the skin resembling scleroderma. In addition, this condition produces disabling contractures, muscles, and tendons. Administration of gadolinium-containing contrast agents is considered a trigger for nephrogenic systemic fibrosis (64). Therefore, administration of gadolinium-based contrast agents in patients with a glomerular filtration rate less than 30 mL per minute should be avoided.
The diagnosis of uremic encephalopathy may be straightforward in a patient with renal compromise, a glomerular filtration rate less than 10% of normal, and altered mentation. Mild renal insufficiency should not be regarded as sufficient to cause encephalopathy, and other causes must be sought. There is no clear-cut direct correlation between the blood urea nitrogen level and the degree of encephalopathy as rapidity of rise of blood urea nitrogen and comorbidities unique to the individual patient may influence clinical severity. In a patient with advanced renal disease and alteration of mental status, several other clinical conditions should be considered in the differential diagnosis. The clinical features of uremic encephalopathy are nonspecific and do not allow differentiation from the other causes of metabolic encephalopathy. Hypocalcemia, hyperphosphatemia, hyperkalemia, metabolic acidosis, and hepatic insufficiency may all be present in a patient with renal failure and may independently result in encephalopathy, requiring specific management. Patients with both hepatic and renal failure are particularly prone to encephalopathy, as the diseased kidney may not be able to adequately clear accumulated plasma ammonia. In severe liver disease, the serum urea and creatinine may not adequately reflect renal function. Diseases affecting the kidney frequently involve other tissues, including those of the CNS, resulting in encephalopathic symptoms that are independent of the consequences of renal failure. For example, systemic lupus erythematosus may result in renal failure that is insufficiently severe to cause encephalopathy but may also give rise to a fulminating CNS vasculitis. Renal failure is often associated with compromise of the immune system, rendering such patients liable to infection, including that of the brain or meninges. Altered mentation from such an infective source may be amenable to treatment if recognized and not attributed to underlying renal dysfunction. Subdural hematoma is a frequently missed cause of encephalopathy and has been reported in 1% to 3.3% of patients undergoing hemodialysis. Drugs that are excreted or significantly metabolized by the kidneys may accumulate in renal insufficiency and result in a toxic encephalopathy. In this case, dialysis may also clear the drug from the blood and will not serve to distinguish this cause of encephalopathy. Cephalosporins can cause nonconvulsive status epilepticus in patients with renal failure, and the clinical picture may resemble uremic encephalopathy (51; 21).
The diagnostic workup in a patient with renal insufficiency who manifests with the features of encephalopathy is directed at (1) defining the cause of renal pathology and its severity, recognizing that mild renal insufficiency is not sufficient to cause alteration in mentation, and (2) the exclusion of other possible causes of encephalopathy. Laboratory tests include measurement of the serum blood urea nitrogen and creatinine and calculation of creatinine clearance. Other commonly found plasma abnormalities include hypocalcemia, hyperphosphatemia, anemia, hyperkalemia, and metabolic acidosis. Evaluation of kidney size (by renal ultrasound, CT, or MRI) will support the diagnosis of established renal failure if the kidney size is small. A renal biopsy is often necessary to establish a definitive pathology and to identify reversible etiologies.
The second directive, that of excluding other possible causes of encephalopathy, will be guided by the clinical presentation but may importantly include screening for other metabolic derangements, particularly hepatic failure, drug toxicity, and infection. The EEG typically shows a generalized excess of slow-wave activity and may show triphasic waves; these features are not characteristic and are not helpful in distinguishing from other causes of metabolic encephalopathy.
MRI in patients with reversible posterior leukoencephalopathy syndrome exhibits white matter signal abnormalities in parieto-occipital regions. Reversible posterior leukoencephalopathy syndrome may affect the brainstem predominantly, and these cases are designated as hypertensive brainstem encephalopathy (23).
White matter hyperintensities have been associated with increased risk of stroke, cognitive decline, and dementia. Chronic kidney disease is a risk factor for vascular disease and has been associated with inflammation and endothelial dysfunction, which have been implicated in the pathogenesis of white matter hyperintensities (32). One third of middle-aged patients with chronic kidney disease have been shown to have silent cerebral white matter lesions. White matter lesions, possibly, reflect ischemic brain damage caused by generalized vascular damage (52). In another study, cerebral magnetic resonance imaging of peritoneal dialysis patients without evidence of cerebrovascular disease revealed a high prevalence of leukoaraiosis of the brain. Old age, poorly controlled hypertension, and the peritoneal dialysis procedure itself or end-stage renal disease were thought to be associated with the occurrence of leukoaraiosis (35). In a multivariate analysis, creatinine clearance 15 to 60 milliliter/min was associated with increased log-white matter hyperintensity volume. Serum creatinine, per 1 mg/dL increase, was also positively associated with log-white matter hyperintensity volume (32).
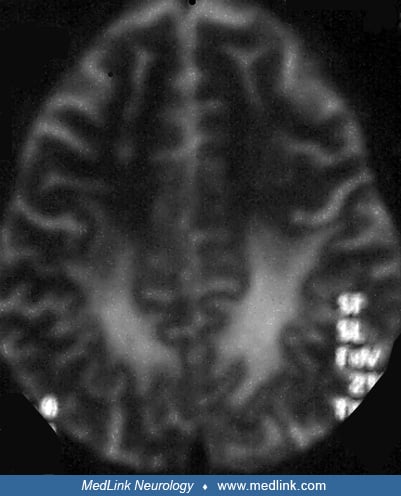
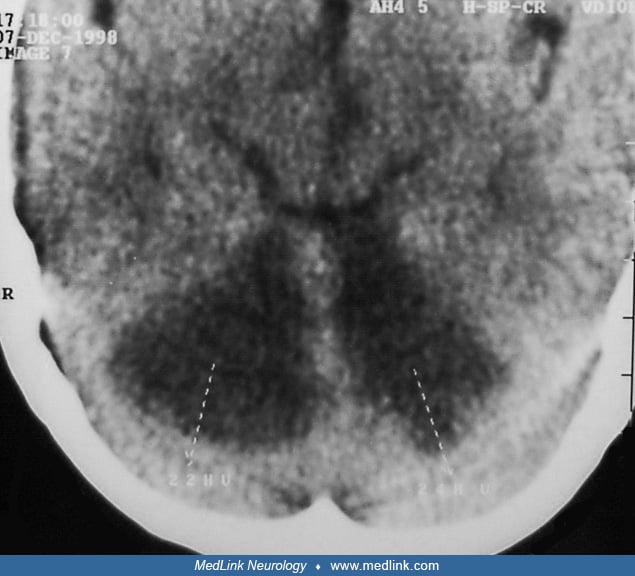
The Lentiform Fork sign is a unique radiographic abnormality that is seen in patients with uremic encephalopathy (55). MRI brain shows T2/FLAIR hyperintensities in the basal ganglia, with a brightly hyperintense rim delineating the medial and lateral borders of the putamina, giving it the appearance of a fork. It has been proposed that metabolic acidosis may be the key factor in the pathogenesis of this sign (43). The lentiform fork sign is a reliable sign for the early diagnosis of uremic encephalopathy. In some patients, follow-up MRI after hemodialysis shows complete resolution of basal ganglion lesions (36; 71).
In patients with uremic neuropathy, electrophysiological assessment reveals decreased compound motor and sensory nerve action potential amplitudes, prolonged conduction velocities, and abnormal late responses typical of a sensorimotor axonal polyneuropathy. Tibial motor nerve conduction studies including F waves, sural sensory nerve action potential amplitude, and vibratory detection threshold are felt to be the most sensitive electrodiagnostic parameters in the diagnosis of uremic polyneuropathy in hemodialysis patients (44). Autonomic nerve tests reveal dysautonomia by reduced R-R interval variation and delayed or absent sympathetic skin response.
Definitive management of uremic encephalopathy is affected by dialysis, renal transplantation, or successful resolution of the renal injury. Acute hemodialysis will relieve the symptoms of uremic encephalopathy within a matter of hours.
In the circumstance where seizures occur in uremic encephalopathy, phenytoin is the most commonly used antiepileptic agent. Phenytoin is highly protein-bound, but in uremia, decreased plasma protein binding increases the fraction of unbound (free), active drug (approximately 25% compared with approximately 8%). The free phenytoin level (about one tenth of the usual total phenytoin level; therapeutic range 1 to 2 µg/mL) is best to monitor dosage, usually given three times a day because of the reduced half-life. Phenytoin is not removed by dialysis, and toxicity in uremic encephalopathy is, thus, difficult to control. Phenobarbital is also frequently used to control seizure activity. Valproic acid and clonazepam can be useful for myoclonus.
Pharmacologic treatment of restless legs syndrome includes dopaminergic agents, opioids, benzodiazepines, and anticonvulsants. The symptoms of uremic polyneuropathy stabilize with long-term hemodialysis, but they improve only in relatively few patients. Ferdousi and colleagues observed that there was no change in uremic neuropathy-related parameters up to 24 months after successful renal transplantation (15). For patients with autonomic neuropathy, specific treatments, including sildenafil for impotence and midodrine for intradialytic hypotension, are effective and well tolerated. Exercise training programs and carnitine supplementation might be beneficial for neuromuscular complications (42).
Vitamin D deficiency is one of the causes of uremic myopathy and treatment with calcitriol can diminish muscle weakness in these patients. Muscle therapies, such as exercise training, are needed in addition to increased oxygen delivery in rehabilitation of dialysis patients. Impaired muscle capillary oxygen transfer has been identified as a pathophysiologic factor, and progressive resistance training has been shown to improve the condition (06).
In general, patients with end-stage renal disease are unable to sustain pregnancy to term. Certain disorders, such as chronic membranous nephropathy and polycystic renal disease, are made worse by pregnancy.
End-stage renal disease is not a contraindication to general anesthesia.
All contributors' financial relationships have been reviewed and mitigated to ensure that this and every other article is free from commercial bias.

Ravindra Kumar Garg DM
Dr. Garg of King George's Medical University in Lucknow, India, has no relevant financial relationships to disclose.
See Profile
Amy A Pruitt MD
Dr. Pruitt of the University of Pennsylvania School of Medicine has no relevant financial relationships to disclose.
See ProfileNearly 3,000 illustrations, including video clips of neurologic disorders.
Every article is reviewed by our esteemed Editorial Board for accuracy and currency.
Full spectrum of neurology in 1,200 comprehensive articles.
Listen to MedLink on the go with Audio versions of each article.
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
General Neurology
Jan. 13, 2025
General Neurology
Jan. 13, 2025
Neuro-Ophthalmology & Neuro-Otology
Jan. 08, 2025
Neuro-Ophthalmology & Neuro-Otology
Jan. 07, 2025
General Neurology
Dec. 30, 2024
General Neurology
Dec. 13, 2024
General Neurology
Dec. 13, 2024
Neuromuscular Disorders
Dec. 09, 2024