Stroke & Vascular Disorders
Fusiform and dolichoectatic aneurysms
Feb. 08, 2025
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Worddefinition
At vero eos et accusamus et iusto odio dignissimos ducimus qui blanditiis praesentium voluptatum deleniti atque corrupti quos dolores et quas.
Acute myocardial infarction is associated with a low but significant risk of stroke. A relationship between unrecognized myocardial infarction and the risk of stroke has been suggested. An analysis of a total of 11,622,528 American patients with acute myocardial infarction recorded 183,896 (1.6%) patients with concomitant acute ischemic stroke. Out of 1,842,529 acute ST-elevation myocardial infarction patients, 22,268 (1.2%) had concomitant acute ischemic stroke. Similarly, out of a total of 11,622,528 patients with acute myocardial infarction, 23,422 (0.2%) had simultaneous intracranial hemorrhage. Most strokes complicating acute myocardial infarction in patients not receiving thrombolysis are cardioembolic. Stroke in patients with acute myocardial infarction adversely affects the outcome. In patients with coronary artery disease, stroke is also associated with a marked increase in the risk of other vascular events like myocardial infarction or stroke (including both ischemic and hemorrhagic stroke), in addition to the risk of death. Approximately 9% of patients with ischemic stroke have silent myocardial infarction. New-onset atrial fibrillation in patients with acute myocardial infarction not only increases the risk of ischemic stroke but also enhances the risk of mortality. Left middle cerebral artery infarction with insular involvement was likely to cause transient cardiac dysfunction and elevated cardiac enzymes. New-onset atrial fibrillation was significantly higher in patients with right middle cerebral artery infarction stroke involving the insula. Thrombolysis-related intracerebral hemorrhage markedly increases the risk of death and disability. Common locations of intracranial hemorrhage are intracerebral, subdural, subarachnoid, and intraventricular. There is a significantly increased risk of vascular dementia after myocardial infarction. The risk is much higher in patients who have experienced stroke after myocardial infarction. It is extremely important to identify stroke-prone patients after myocardial infarction and to institute appropriate preventive measures. Increased ticagrelor (a platelet aggregation inhibitor) usage in the management of acute myocardial infarction has led to a decreased incidence of ischemic stroke. Two studies identified risk factors for stroke in patients following post-coronary artery bypass grafting: longer surgery times, myocardial infarction, cardiogenic shock, peripheral vascular disease, age, and surgical year. Recognizing these risks is crucial for better outcomes. In some preliminary reports, cangrelor infusion has also shown promise. In this article, the author has provided the latest information available on this subject.
|
• Stroke can complicate the course of acute myocardial infarction. | |
|
• Ischemic strokes are the predominant type of stroke seen in non-ST-segment elevation acute myocardial infarction. | |
|
• Most ischemic strokes complicating acute myocardial infarction are cardioembolic. | |
|
• Intracerebral hemorrhage can occur after thrombolysis for acute myocardial infarction. | |
|
• Development of stroke is one of the major reasons for mortality after coronary artery bypass operations. | |
|
• Acute myocardial infarction is also an important medical complication of ischemic stroke. | |
|
• Aspirin, anticoagulants, and early coronary revascularization diminish the risk of ischemic stroke with acute myocardial infarction. |
Since the early 19th century, several authors have noted the occurrence of mural thrombi complicating acute myocardial infarction. Virchow postulated that three factors predispose patients to thrombosis: (1) injury of the vascular endothelial or endocardial surface, (2) circulatory stasis, and (3) a generalized hypercoagulant state (100). He reported occlusion of arteries in the brain by thrombi, which seemed to have originated in the heart, and named this phenomenon "embolism" (from the Greek word for “plug”).
Gordinier suggested that the sudden arterial plugging of the vessels of the brain, viscera, or extremities indicates involvement of a branch of the left coronary, whereas signs of pulmonary infarct suggest involvement of the right coronary or its branches (33). Blumer was among the first to extensively discuss the importance of embolism as a complication of cardiac infarction. He stated that mural thrombi are common following cardiac infarction and that fragments may detach and produce embolic phenomena (15).
Stroke can complicate the course of patients presenting with acute myocardial infarction. Ischemic strokes are the predominant type of stroke seen in patients with non-ST-segment elevation acute coronary syndromes, whereas intracerebral hemorrhage constitutes a significant proportion of strokes after thrombolysis for acute ST-segment elevation myocardial infarction (49).
Stroke may manifest in many ways according to the part of brain involved. Clinical features are insufficiently predictive to reliably distinguish cerebral infarction from hemorrhage or thrombotic from embolic occlusion. Features suggestive of embolic stroke, in addition to the presence of a recent myocardial infarction or left ventricular thrombi, are abrupt onset, headache, seizures, evidence for deficits in multiple vascular territories, and embolization to other organs. Most ischemic strokes after acute myocardial infarction involve the anterior circulation and are nonlacunar (71). Posterior circulation strokes are unusual. Approximately one third occur within 24 hours following admission, whereas about two thirds occur in the first week after the myocardial infarction (12; 87).
Symptomatic intracerebral hemorrhage, the most dreaded complication of thrombolytic therapy, usually occurs during the first 2 days after thrombolysis (34; 37). Patients usually develop a change in the level of consciousness, headache, nausea, and vomiting in addition to specific focal neurologic deficits depending on the location of the bleeding (86). Onset can be catastrophic and rapidly fatal.
There is growing evidence to suggest that cerebral amyloid angiopathy, which itself can cause hemorrhage, may be a risk factor for thrombolysis-related intracerebral hemorrhage. Cerebral amyloid angiopathy and thrombolysis-related intracerebral hemorrhage have several similar clinical features, such as a predisposition to lobar or superficial regions of the brain, multiple hemorrhages, increasing frequency with age, and an association with dementia (65).
There is a significantly increased risk of vascular dementia after myocardial infarction. The vascular dementia risk is much higher in patients who have experienced stroke after myocardial infarction (92).
Cardiocerebral infarction is defined as the simultaneous occurrence of both acute myocardial infarction and acute stroke. A systematic review by Ng and colleagues recorded 44 such cases (76). In this series, 15 patients were treated with percutaneous coronary intervention with a stent, and eight patients were treated without a coronary stent. Cerebral vessel thrombectomy was done in 10 patients, and eight patients were treated with thrombectomy of coronary vessels. Medically, patients were treated with thrombolytics, anticoagulants, or antiplatelet drugs. Ten patients died. The authors concluded that cardiocerebral infarction is associated with substantially poor outcomes. Ruiz-Ares and colleagues described two patients with cardiocerebral infarction following COVID-19 (81).
Mortality after acute myocardial infarction complicated by an ischemic stroke is very high. Data for 173,233 unselected patients with acute myocardial infarction from the Swedish Heart Intensive Care Admissions registry for 1998 through 2008 revealed the 1-year mortality was 36.5% for acute myocardial infarction complicated by ischemic stroke and 18.3% for acute myocardial infarction without stroke (17). In patients with coronary artery disease, stroke is also associated with a marked increase in the risk of other vascular events like myocardial infarction or stroke (including both ischemic and hemorrhagic stroke), in addition to the risk of death. The excess risk of hemorrhagic stroke is particularly high in patients receiving dual antiplatelet therapy (aspirin and clopidogrel) and in the first year after stroke/transient ischemic attack (26). Previous myocardial infarction has also been associated with poorer stroke outcomes. A 2008 study assessed the effect of previous myocardial infarction on the characteristics and outcome of stroke (11). In this study of 4190 patients with stroke, 460 (11%) reported a history of myocardial infarction. Contrary to earlier experience in this study, the acute neurologic state and the 28-day mortality did not differ between the two groups. Compared with patients without previous myocardial infarction, those with myocardial infarction were significantly older, more often males, smokers, alcohol consumers, and had a more severe pre-stroke level of handicap. Patients with a history of myocardial infarction more frequently had atrial fibrillation and a history of transient ischemic attack.
Nazir and colleagues, in a large study, noted a poor prognosis in stroke patients having a concomitant type-2 myocardial infarction (75). Among 587,550 acute ischemic stroke patients included, 4182 (0.71%) had type-2 myocardial infarction. Acute ischemic stroke patients with type-2 myocardial infarction had significantly higher in-hospital mortality, poor functional outcome, more hospital costs, higher rate of discharge to a facility, increased length of hospital stay, and higher rate of 30-day all-cause readmissions.
Although thrombolytic therapy has little effect on overall stroke occurrence, it increases the risk of stroke death substantially (57). In the GUSTO mega-trial, 60% of patients with primary intracerebral hemorrhage died, and 25% were disabled, versus 17% dead and 40% disabled with nonhemorrhagic infarcts. In a quality-of-life study, patients with moderate or severe residual deficits showed significantly decreased quality of life (34). Strokes were associated with a 60% increase in cumulative 1-year medical costs, and follow-up costs were more than quadrupled for stroke survivors, dominated by the cost of institutional care (97).
Cerebrovascular accidents after percutaneous coronary interventions, although rare, are associated with high rates of in-hospital death and acute renal failure, often requiring dialysis (27). Perioperative stroke is also associated with a substantially increased risk of postoperative death among coronary artery bypass grafting surgery patients. The greatest risk of death was noted within the first year after surgery. Survival after 1 year approximates that of patients who did not suffer a stroke (24).
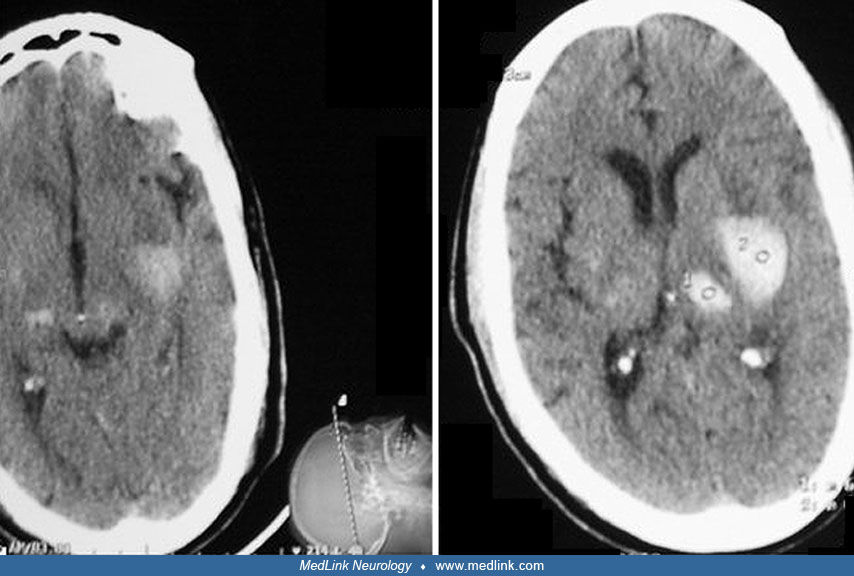
A 55-year-old, right-handed, white male with a medical history of hypertension and diabetes presented to the emergency room after 2 hours of severe retrosternal pain radiating to the left arm and associated with diaphoresis. The initial electrocardiogram was compatible with an acute onset of an anteroseptal wall myocardial infarction. The patient was treated with aspirin and intravenous streptokinase; subcutaneous heparin therapy was started later. Forty-eight hours after completion of streptokinase therapy, he developed nonfluent aphasia and right hemiparesis (more severe in the face and upper extremities). Brain CT revealed subtle early infarct changes in the left frontal lobe with no evidence of blood. On transthoracic echocardiography, no left ventricular thrombus was seen, but significant anterior wall motion abnormalities were identified. Carotid duplex demonstrated 0% to 15% stenosis in both internal carotid arteries. Over the next 48 hours, the neurologic deficit improved substantially. A repeat brain CT did not reveal any secondary hemorrhagic conversion, and the patient was discharged on oral anticoagulants for secondary prevention of an embolic stroke.
It is not exactly clear why patients have ischemic strokes after acute coronary syndromes. The association between coronary artery disease and stroke can be ascribed to a common pathophysiologic process, atherosclerosis. One possible cause is the formation of mural thrombi in areas of ventricular hypokinesis after myocardial damage. These mural thrombi may subsequently dislodge and embolize the cerebral vasculature. Atrial fibrillation and cardioversion may also be associated with embolization of thrombi from the left atrium (49). In a study, new-onset atrial fibrillation during acute myocardial infarction was associated with a long-term risk for stroke, and these patients may potentially benefit from anticoagulant therapy (68). New-onset atrial fibrillation in patients with acute myocardial infarction not only increases the risk of ischemic stroke but also enhances the risk of mortality (42).
In addition to cardiac arrhythmias, a decrease in stroke volume during the acute phase of myocardial infarction can reduce cardiac output to such an extent that hemodynamic compromise results. Strokes occurring several weeks after myocardial infarction may be due to chronic left ventricular thrombi, an akinetic left ventricular segment, or left ventricular dysfunction. Indeed, cerebral microembolism was detected by transcranial Doppler more often among patients with acute myocardial infarction with reduced left ventricular function, akinetic segments, or left ventricular thrombi (73). For every decrease of 5% in the ejection fraction, an 18% increase in the risk of long-term stroke has been found (56). Other potential cardiac causes of stroke, as well as atherothrombotic large vessel disease and small vessel disease, must also be considered (64). It may be difficult to determine the exact mechanism of stroke in many patients, in which different pathophysiologic mechanisms may be equally likely.
Left ventricular thrombi occur frequently in the course of acute myocardial infarction and are associated with an increased embolic risk (21). Although 2-dimensional echocardiography has some limitations, it is a fairly accurate noninvasive method for detecting left ventricular thrombi.
Left ventricular thrombi develop in up to one third of patients with anterior myocardial infarction but only in 0% to 5% of patients with inferior infarctions. The highest rate of occurrence of left ventricular thrombi is found in patients with anterior infarcts and a low ejection fraction or congestive heart failure (21). Overall, the published data suggest that cerebral embolism occurs clinically within the first 3 months after myocardial infarction in about 10% of those with echocardiographically evident left ventricular thrombi, although it is often difficult to classify acute ischemic events as embolic in patients with advanced atherosclerotic disease. Mobile thrombi, or those that protrude into the left ventricular cavity, appear to have the highest embolic risk.
The occurrence of intracranial hemorrhage complicating acute myocardial infarction was considered a rare event before the thrombolytic era. Intracranial hemorrhage alone, associated with treatment of acute myocardial infarction, is mostly coagulopathy-induced and is associated with thrombolytic therapy or in combination with other antithrombotic drugs (85; 32). The incidence, predictors, or outcomes of intracranial hemorrhage in patients with non-ST-segment elevation acute coronary syndromes are still not exactly known. Data from four antithrombotic therapy trials revealed that of 37,815 patients, 135 (0.4%) had an intracranial hemorrhage. Patients with older age, prior transient ischemic attack/stroke, higher systolic blood pressure, or larger number of antithrombotic agents were at increased risk. One third of patients with intracranial hemorrhage died. The locations of intracranial hemorrhage were intracerebral, subdural, subarachnoid, and intraventricular (61). Another study noted that the yearly incidence of intracranial hemorrhage after acute myocardial infarction is approximately 0.35% (36). Advanced age, decreased renal function, previous ischemic or hemorrhagic stroke, and anticoagulant therapy predicted an increased risk of intracranial hemorrhage.
Paradoxical embolic complications of intravenous thrombolysis during tissue plasminogen activator infusion for acute ischemic stroke are increasingly recognized, and several cases of myocardial infarction soon after the administration of alteplase have been reported (63; 106).
Acute myocardial infarction is an important medical complication of ischemic stroke as well (52). A review of 39 cohort studies reported an annual linear risk of about 2% for both myocardial infarction and nonvascular death in patients with transient ischemic attack and strokes (96). One study evaluated the frequency and clinical impact of in-hospital myocardial infarction following acute ischemic stroke (54). In this study, 9180 patients with acute ischemic stroke were included. Overall, 211 (2.3%) patients were reported to have myocardial infarction during hospitalization. At hospital discharge, 64.9% of patients with in-hospital myocardial infarction had died or were severely disabled, compared with 35.8% in the entire cohort. Patients with previous coronary artery disease, diabetes, peripheral vascular disease, and severe stroke had a higher risk of myocardial infarction early during stroke recovery. A study noted that out of 864,043 acute ischemic stroke patients, 13,573 patients (1.6%) had a myocardial infarction (79.5% non-ST-segment-elevation and 20.5% ST-segment-elevation). The predictors of myocardial infarction were older age, history of coronary artery disease, chronic renal insufficiency, undergoing mechanical thrombectomy, and rhythm and conduction abnormalities. In-hospital mortality was 21.4% and 7.1% in patients with and without myocardial infarction, respectively (04). In a large population-based study, first-ever ischemic stroke predicted an increased risk of incident major adverse cardiovascular events that included myocardial infarction, unstable angina, congestive heart failure, coronary artery disease, coronary artery revascularization, or cardiovascular death (91). The authors hypothesized that associated cardiac injury, preexisting subclinical cardiovascular disease, or both might be responsible. In a study of 115 patients with acute ischemic stroke, cardiac MRI revealed that over one third had focal myocardial fibrosis, often with an ischemic pattern. This was more prevalent in those with diabetes, prior myocardial infarction or stroke, and elevated troponin levels. Fibrosis was associated with diffuse myocardial changes and reduced strain in heart muscle function. These findings, potentially indicative of acute or subacute conditions, underscore the need for further research to understand their long-term impact on acute ischemic stroke prognosis (14).
The evidence from primary prevention of myocardial infarction studies suggests that there is an increased risk for hemorrhagic stroke associated with the use of aspirin. This risk of hemorrhagic stroke is approximately 0.2 events per 1000 patient-years, which is comparable to estimates of the risk associated with the use of aspirin in secondary prevention patients (35). Orbofiban is an oral glycoprotein IIb/IIIa receptor inhibitor. The integrin glycoprotein IIb/IIIa receptor is the final common pathway to platelet aggregation. In the Orbofiban in Patients with Unstable Coronary Syndromes--Thrombolysis In Myocardial Infarction (OPUS-TIMI) 16 study, a multicenter, randomized, placebo-controlled trial, patients were randomized to aspirin plus either orbofiban or placebo and followed for up to 1 year. Cerebrovascular events were prospectively identified and classified. During 10 months of follow-up, 150 (1.5%) patients experienced cerebrovascular events. The overall incidence of cerebrovascular events after acute coronary syndromes was highest in the first 30 days and then declined. Compared with placebo, treatment with orbofiban was associated with a nonsignificant increased risk of ischemic stroke or transient ischemic attack. It was concluded that orbofiban was not effective in preventing ischemic stroke or transient ischemic attack (88).
Most strokes complicating acute myocardial infarction in patients not receiving thrombolysis are likely cardioembolic, as suggested by their association with large anterior myocardial infarcts and left ventricular thrombi detected by echocardiography. Left ventricular thrombi usually occur when the anterior wall or apex becomes akinetic or hypokinetic, enlarging the apical zone of intraventricular stasis. Inflammatory changes at the endocardial surface also enhance thrombogenicity. A systemic hypercoagulable state may promote thromboembolism early after the coronary event, whereas residual fresh thrombus may enhance coagulation during the first 1 to 3 months. The risk of systemic embolization from left ventricular thrombi depends on the balance between factors favoring thrombus formation within the ventricular cavity (endocardial injury, regional circulatory stasis, and activation of the intrinsic coagulation system) and dynamic forces of the circulation that may project thrombotic material into the systemic circulation, producing clinical morbidity. Cardioembolic strokes have a propensity for secondary hemorrhagic transformation. Hemorrhagic transformation may occur either because of distal migration of embolic fragments with reperfusion of infarcted tissue or because of collateral circulation. Disruption of an atherosclerotic plaque from the aortic arch during catheter or surgical manipulation could act as a source of emboli during invasive procedures such as cardiac catheterization, percutaneous coronary intervention, intra-aortic balloon pumping, and coronary artery bypass surgery (49). It has been suggested that the presence of heart failure on admission for an acute myocardial infarction increases in-hospital stroke risk. The study has further suggested that heart failure treatments may modify the risk of stroke (93). Sotomi and colleagues noted that percutaneous coronary intervention was associated with an enhanced risk of stroke (90). A total of 9147 patients who underwent primary percutaneous coronary intervention within 24 hours of hospitalization were evaluated. Thrombus aspiration was done in 4448 patients and not in the remaining 4699 patients. Logistic regression analysis revealed that the thrombus aspiration procedure was independently associated with a significant increase in 7-day stroke risk.
Hypertensive intracerebral hemorrhages are classically located in the distribution of perforating vessels and are associated with numerous microvascular lesions, such as Charcot-Bouchard microaneurysms. With thrombolytic therapy, intracerebral hemorrhages are often large, lobar, and supratentorial, occasionally in multiple compartments and with fluid levels inside the hematoma (31; 65). Accordingly, other underlying conditions may contribute to the cause of bleeding, such as preexisting vascular malformations and, probably more important in an elderly population, cerebral amyloid angiopathy (86). Diverse hemostatic derangements, depending on the thrombolytic agent used, dose, protocol of administration, and adjunctive therapy, contribute to the bleeding tendency.
Diffuse myocardial damage characterized by myocardial necrosis and subendocardial hemorrhages has been reported to occur after stroke. This type of injury is called myocytolysis and results from intense sympathetic nervous system activation (09). Asymptomatic hypertroponinemia following acute ischemic stroke, without apparent myocardial infarction, is associated with an increased long-term mortality. In a study that included 1999 acute ischemic stroke cases, 706 (85.3%) had a cardiac troponin drawn, and 1590 (79.5%) had echocardiograms (105). Hypertroponinemia occurred in 353 of 1706 cases (20.7%), and 160 of 1590 cases (10.1%) had abnormal echocardiograms. Hypertroponinemia was associated with increased mortality at 3 years.
Acute stroke can alter central autonomic functions, resulting in myocardial injury, electrocardiographic abnormalities, cardiac arrhythmias, and, ultimately, sudden death. Experimental and clinical evidence suggests that autonomic imbalance is more frequent after infarcts involving the insular cortex, a crucial region for the control of sympathetic and parasympathetic autonomic functions (89). One study suggested that parietal lobe infarction is an independent predictor of long-term cardiac death or myocardial infarction in patients with stroke (80). In this study, first ischemic stroke patients were prospectively followed up for cardiac death, defined as fatal myocardial infarction, fatal congestive heart failure, or sudden death or arrhythmia, and for nonfatal myocardial infarction. Of these, fatal myocardial infarction occurred in 38.6%, fatal congestive heart failure in 18.2%, and sudden death in 43.2%. In multivariate models, clinical diagnosis of left parietal lobe infarction was associated with cardiac death or myocardial infarction. Min and colleagues demonstrated that acute left middle cerebral artery infarction with insular involvement was likely to cause transient cardiac dysfunction and elevated cardiac enzymes without adversely affecting outcomes (69). The incidence of new-onset atrial fibrillation was significantly higher in patients with right middle cerebral artery infarction stroke involving the insula. However, there was no increased risk of recurrent ischemic stroke in patients with newly developed atrial fibrillation. Buckley and colleagues also demonstrated that newly-diagnosed cardiovascular complications following an ischemic stroke were very common and post-stroke cardiovascular complications were associated with a poor 5-year outcome (19). Authors noted that among 365,383 patients with stroke with 5-year follow-up, 11.1% had acute coronary syndrome, 8.8% atrial fibrillation/flutter, 6.4% heart failure, 1.2% ventricular arrhythmias, and 0.1% Takotsubo syndrome, all within 4 weeks of occurrence of ischemic stroke.
The development of carotid atherosclerosis closely parallels coronary atherosclerosis. Many patients with clinically apparent or silent myocardial ischemia have coexistent cerebrovascular disease. Conversely, many patients with cerebrovascular disease have various degrees of coronary artery disease. Indeed, myocardial infarction is the leading cause of death in patients who recover from strokes or transient ischemic attacks. The incidence of stroke during the acute phase following myocardial infarction varies considerably between studies. Rates are mostly in the range of 0.8% to 3.2%. For example, in one such study, Kassem-Moussa and colleagues reported the incidence and characteristics of stroke during 90-day follow-up in patients stabilized after an acute coronary syndrome (49). Of 15,904 stabilized patients with acute coronary syndrome, 113 (0.71%) had a stroke over a median follow-up of 90 days. The majority of strokes occurred within 30 days of presentation. Of the 113 strokes, 88 (78%) were ischemic, 13 primary hemorrhagic, two primary ischemic with hemorrhagic conversion, and 10 patients had stroke of uncertain type. Of patients who had a stroke, 52% died or had moderate or severe disability. Patients with stroke were older and more often had hypertension, diabetes, peripheral vascular disease, and atrial fibrillation. Among patients with stroke who had cardiac catheterization, percutaneous coronary intervention, or coronary artery bypass grafting, stroke occurred predominantly after the procedure. On multivariable analysis, age, heart failure, prior stroke, left bundle branch block, and systolic blood pressure predicted the occurrence of stroke. In the VALIANT trial, data from 14,703 patients with high-risk myocardial infarction revealed that 463 (3.2%) patients had fatal (n = 124) or nonfatal (n = 339) strokes, with 134 strokes occurring in the first 45 days. In patients with acute myocardial infarction complicated by heart failure, left ventricular systolic dysfunction, or both, diastolic blood pressure higher than 90 mmHg, prior stroke, and atrial fibrillation were the most powerful predictors of stroke. Ejection fraction and sex were not predictive of stroke in this cohort (83). Data from the Worcester Heart Attack Study indicated an incidence of 1.4% (82). Of 9220 patients without a history of previous stroke hospitalized with confirmed acute myocardial infarction, 132 experienced an acute stroke during hospitalization. Advanced age, female sex, a previous myocardial infarction, and the occurrence of atrial fibrillation during hospitalization were associated with a greater risk of stroke. Receipt of a percutaneous coronary intervention during hospitalization was associated with a lower risk of stroke. In the United States, among the 1,924,413 patients admitted for acute myocardial infarction, the overall rate of cerebrovascular accidents was 2% (ischemic stroke: 1.47%, transient ischemic attack: 0.35%, and hemorrhagic stroke: 0.21%). Female gender, older age (age ≥ 65), black ethnicity, comorbidities including chronic renal failure, peripheral vascular diseases, atrial fibrillation as well as ST-elevation myocardial infarction, and coronary artery bypass graft were associated with the highest risk of cerebrovascular accident post-myocardial infarction (74). Ischemic stroke is a more common complication after an acute myocardial infarction in chronic kidney disease patients than in nonchronic kidney disease patients (47).
Aggarwal and colleagues analyzed huge data (from the years 2000 to 2017) using the National Inpatient Sample database and noted that out of a total of 11,622,528 acute myocardial infarction admissions, 183,896 (1.6%) had concomitant acute ischemic stroke (01). As compared with the year 2000, in 2017, the incidence of acute ischemic stroke increased slightly in patients with ST-segment-elevation acute myocardial infarction. Stroke incidence decreased in non-ST-segment-elevation acute myocardial infarction. Old age, female sex, non-White race, comorbidities, and cardiac arrhythmias predicted acute ischemic stroke following acute myocardial infarction. The acute ischemic stroke cohort had higher in-hospital mortality, longer hospital length of stay, higher hospitalization costs, greater use of tracheostomy and percutaneous endoscopic gastrostomy, and less frequent discharges to home. Among acute ischemic stroke survivors, 57.3% had a poor functional outcome at discharge (01). Albaeni and colleagues analyzed the same database and noted that out of 1,842,529 acute ST-elevation myocardial infarction patients who were hospitalized from 2003 to 2014, 22,268 (1.2%) had an acute ischemic stroke (02). Old age, female sex, atrial fibrillation, and heart failure predicted strokes in acute ST-elevation myocardial infarction patients. Patients with ischemic strokes had higher in-hospital mortality following acute ST-elevation myocardial infarction. Patlolla and colleagues analyzed the data of intracranial hemorrhage following acute myocardial infarction and noted that out of 11,622,528 acute myocardial infarction admissions, 23,422 (0.2%) patients had concomitant intracranial hemorrhage (78). Old age, female sex, non-White race, multiple comorbidities, cardiac arrhythmias, use of fibrinolytics, mechanical circulatory support, and invasive mechanical ventilation were predictors of intracranial hemorrhage. Intracranial hemorrhage following acute myocardial infarction carried a poor outcome. In a retrospective study involving 8,049 patients with acute coronary syndrome from 2007 to 2018, risk factors for ischemic stroke post-acute coronary syndrome were analyzed. Key risk factors for early- and late-onset ischemic stroke included previous stroke, atrial fibrillation or flutter, heart failure, lower left ventricular ejection fraction, severe coronary artery disease, older age, and peripheral artery disease. Particularly, a CHA2DS2-VASc score of 6 or higher significantly heightened the risk of both early- and late-onset ischemic stroke, underscoring its value in predicting stroke risk after acute coronary syndrome (44).
Stroke is a frequent complication in patients with acute myocardial infarction treated invasively. Data on long-term follow-up of 2520 patients were screened to identify patients who had a stroke (79). During a median follow-up of 25.5 months, 52 patients (2.07%) had a stroke. Previous stroke, female sex, glomerular filtration rate lower than 60 ml/min/1.73 m, and contrast nephropathy were predictors of stroke.
A study by Albaker and colleagues evaluated early stroke following acute myocardial infarction (03). The incidence of in-hospital stroke following acute myocardial infarction was 0.85% among 5,833 consecutive patients who were enrolled from 64 hospitals in six Middle East countries. Independent predictors of stroke were age, prior stroke, prior coronary artery bypass surgery, anterior acute myocardial infarction, and systolic blood pressure higher than 190 mm Hg on presentation. Early administration of statins was independently associated with reduced stroke risk.
A large proportion of myocardial infarction in the elderly remains unrecognized and is identified only by electrocardiography. The relationship between unrecognized myocardial infarction and the risk of stroke was evaluated. The authors found that unrecognized myocardial infarction was associated with a strongly increased risk of stroke in men. The risk increase was largest for ischemic strokes, particularly cortical ischemic strokes. This suggests that screening the elderly for unrecognized myocardial infarction using electrocardiography can contribute to identifying persons at increased risk of stroke (46).
Late stroke following myocardial infarction is infrequent. Herlitz and colleagues assessed the factors associated with the development of stroke during long-term follow-up after myocardial infarction (41). The study was performed in 31 hospitals in Sweden. The mean follow-up was 5 years, with a range of 1.7 to 6.7 years. In all, 3300 patients were randomized in this study, of which approximately 6% (194) of patients developed stroke (4.2% nonhemorrhagic, 0.5% hemorrhagic, and 1.3% uncertain). Risk indicators for stroke long-term after myocardial infarction were increasing age, a history of diabetes mellitus, a previous stroke, hypertension, or smoking. The data regarding the occurrence of stroke following myocardial infarction indicate that 2.9% (660 out of 22,904) of patients with myocardial infarction had a stroke within a median follow-up period of 1.9 years (1.3 to 2.7 years). The risk factors were old age, female sex, smoking, and hypertension. Patients with stroke also had a higher Killip class, a lower glomerular filtration rate, and a higher proportion of myocardial infarction, myocardial infarction, diabetes, and stroke in the past (29).
In addition to estimating the rate of stroke after incident myocardial infarction, studies also have examined how the rate of stroke after myocardial infarction changed over time. The impact of stroke on survival after incident myocardial infarction has also been studied. The risk for myocardial infarction is significantly higher among stroke patients than in the general population. Stroke patients with previous coronary heart disease and peripheral artery disease are at the highest risk (08). In a large study, a total of 2160 patients with myocardial infarction were hospitalized between 1979 and 1998 and followed for a median of 5.6 years (range, 0 to 22.2 years) (102). The rate of stroke was 22.6 per 1000 person-months during the first 30 days after myocardial infarction, corresponding to a 44-fold increased risk for stroke. The risk for stroke remained two to three times higher than expected during the first 3 years after myocardial infarction. Older age, previous stroke, and diabetes increased the risk of stroke, which did not decline over the study period. Strokes were associated with a large increase in the risk of death after myocardial infarction. Merkler and colleagues noted that 8.6% (362 of 4224) of patients with ischemic stroke had a silent myocardial infarction (66).
The risk of stroke has decreased in recent years. In a study of 173,233 patients with acute myocardial infarction, 3571 (2.1%) developed ischemic stroke within 30 days. The incidence of ischemic stroke was significantly lower during the years 2007 to 2008 compared with 1998 to 2000. Independent predictors of an increased risk of stroke were age, female sex, prior stroke, diabetes mellitus, atrial fibrillation, clinical signs of heart failure in hospital, ST-segment-elevation myocardial infarction, coronary artery bypass grafting, and angiotensin-converting enzyme inhibitor treatment at discharge. Percutaneous coronary intervention, fibrinolysis, acetylsalicylic acid, statins, and P2Y12 inhibitors were predictors of reduced risk of stroke (48).
In addition to acute myocardial infarction, acute heart failure is also common and associated with increased mortality in stroke patients. In a study of 814 stroke patients, 53 patients (6.5%) had an acute myocardial infarction or acute heart failure; all these events occurred in patients with ischemic stroke (67). At 3 months, 151 patients had died. History of angina, acute myocardial infarction in the 3 months before admission, hyperglycemia, and a high National Institutes of Health Stroke Scale score on admission were associated with in-hospital acute myocardial infarction or acute heart failure.
In the large-scale thrombolytic trials of the past, the overall reported incidence of stroke during hospitalization for acute myocardial infarction was 0.9% to 1.6%, of which intracranial hemorrhage represented 0.2% to 0.9%, depending on the type and dose of agent used (06; 07; 58); however, caution must be exercised in the interpretation of these reports, as recording and evaluation of strokes were not standardized, and variable proportions of strokes were unclassifiable. Furthermore, thrombolytic trials often excluded high-risk individuals (above a certain age, with a history of stroke, or with uncontrolled hypertension), so reported rates may underestimate the true magnitude of this complication. Indeed, in clinical practice, a higher rate of elderly patients with acute myocardial infarction experienced intracranial hemorrhage (37; 18). In a study of 12,739 patients who received fibrinolytic therapy for acute myocardial infarction from 1998 to 2000, 146 patients (1.15%) sustained strokes, and 82 patients (0.65%) had an intracerebral hemorrhage. Advanced age, female sex, history of a past cerebrovascular event, and systolic hypertension on arrival (systolic blood pressure greater than 160 mm Hg) were identified as independent risk factors for intracerebral hemorrhage. Patients receiving streptokinase had a lower risk of intracerebral hemorrhage. Among the patients at high risk for intracerebral hemorrhage, the intracerebral hemorrhage rates remained low, ranging from 0.7% to 1.8%. This study further confirmed that age was the most important predictor of intracerebral hemorrhage in patients receiving fibrinolytic therapy. Patients after myocardial infarction had fewer systemic hemorrhages when treated with tenecteplase compared with recombinant tissue-type plasminogen activator (45).
The overall risk of stroke due to thrombolytic therapy in properly selected acute myocardial infarction patients is low compared with the impressive reduction in mortality and, thus, is associated with a favorable benefit-risk profile. Indeed, the incidence of early cerebrovascular events among unselected patients admitted to coronary care units in the prethrombolytic versus thrombolytic eras remained similar, although mortality from acute myocardial infarction decreased substantially (94). In patients with acute coronary syndromes without persistent ST-segment elevation, the incidence of stroke is low but still associated with substantial morbidity and mortality (62; 23). Patients undergoing early coronary artery bypass grafting surgery, but not early percutaneous coronary intervention, have a substantially increased risk of stroke (23). Nevertheless, many valid clinical reasons advocate performing earlier, rather than delayed, coronary artery bypass grafting surgery. Dacey and colleagues examined the survival of 35,733 consecutive patients undergoing isolated coronary artery bypass graft surgery in northern New England from 1992 through 2001 (24). Perioperative stroke occurred in 575 patients (1.61%). Patients who had strokes had more comorbidity. In patients undergoing coronary artery bypass graft surgery, the independent predictors for stroke, in order of risk, were age older than 70 years, poor preoperative neurologic status, and previous cardiac surgery (104). Elahi and colleagues investigated the validity of the stroke risk index in identifying patients who develop a stroke following coronary artery bypass grafting on cardiopulmonary bypass. Retrospective data were analyzed from 6846 patients. A total of 217 patients (3.2%) with a mean age 65.9 +/- 11.7 years developed adverse neurologic events following surgery. These findings demonstrate that factors such as a preoperative history of redo procedures, myocardial infarction, ejection fraction below 30%, and absence of sinus rhythm play an important role in identifying the at-risk population (28).
Cerebrovascular accidents after percutaneous coronary interventions are rare. In a study population comprising 20,679 patients who underwent percutaneous coronary interventions, cerebrovascular accidents occurred in 92 patients (0.3% of procedures). Of these, transient ischemic attacks occurred in 13 patients (0.04%) and stroke in 79 patients (0.25%) (27). One study used the combined 2000 to 2001 New York State Angioplasty Registry to compare the clinical characteristics and in-hospital outcomes of patients with and without stroke after percutaneous coronary intervention. Of the 76,903 patients who underwent angioplasty, 140 (0.18%) experienced stroke. Multivariate regression analysis suggested age, glycoprotein IIb/IIIa inhibitor use, acute myocardial infarction or congestive heart failure on admission, history of carotid disease, chronic renal disease, and placement of an intra-aortic balloon pump as independent predictors for stroke that complicate percutaneous coronary intervention (103). One study investigated the predictors of acute stroke in 1344 patients undergoing coronary artery bypass grafting surgery for acute coronary syndrome. Factors like longer cardiopulmonary bypass and cross-clamp times and perioperative myocardial infarction were linked to higher stroke risk. Independent predictors identified were cardiogenic shock, peripheral vascular disease, and previous stroke. Recognizing these risks preoperatively is vital for improving outcomes (50). Another study analyzed 20,582 patients who underwent coronary artery bypass grafting from 1998 to 2019 to identify stroke predictors. The incidence of postoperative stroke was 0.7%, with a higher mortality rate in these patients. Factors like age, peripheral arterial disease, re-exploration for bleeding, perioperative myocardial infarction, and the year of surgery were key predictors (51).
A South Korean study demonstrated a substantial risk of acute myocardial infarction following acute stroke (53). Out of 11,720 patients with acute ischemic strokes, the 5-year cumulative incidence of acute myocardial infarction was 2.0%. The annual risk was highest in the first year. The risk of myocardial infarction was highest in the first year, particularly in patients who had a prior history of coronary heart disease. Patients with lacunar infarcts had a much lower incidence compared with large-vessel occlusion or cardioembolic strokes.
Analysis of Chinese data revealed that the presence of atrial fibrillation substantially increases the risk of stroke following myocardial infarction. A retrospective study included data from 170,472 patients; 8530 patients had atrial fibrillation (20). A matching technique identified 8530 patients without atrial fibrillation. The 12-year risk of stroke was higher in patients with atrial fibrillation than in those without atrial fibrillation. In addition, those with preexisting atrial fibrillation had an even higher risk of stroke when compared with those who had newly diagnosed atrial fibrillation.
An optimal strategy for managing this potentially catastrophic complication of myocardial infarction includes the identification of stroke-prone patients and the institution of preventive measures. The main predictors of stroke after acute myocardial infarction are older age, higher heart rate, diabetes, hypertension, anterior site of myocardial infarction, high cardiac enzyme levels, impaired left ventricular function, atrial arrhythmias, coronary angiography or bypass surgery, lack of aspirin use, and prior cerebrovascular disease, peripheral vascular disease, or angina (12; 70; 59; 55; 49). These data are in accord, in part, with findings that left ventricular thrombi more commonly complicate anterior infarctions and that these thrombi, especially if protruding and mobile, are a source of embolization. A useful and simple scoring monogram for risk assessment in the acute phase was developed based on the results of the Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO-1) trial (59). The strongest predictor identified for late stroke following myocardial infarction is chronic atrial fibrillation. A risk stratification score was constructed from the Cooperative Cardiovascular Project for elderly patients discharged from the hospital after an acute myocardial infarction. The 6-month stroke admission rate for patients with a score of 4 or higher (equaling 20% of the total sample) was equal to 4% (55). Transient atrial fibrillation complicating acute myocardial infarction is associated with an increased future risk of ischemic stroke and transient ischemic attacks, particularly in patients treated with antiplatelet agents alone. High atrial fibrillation recurrence rates in these patients also suggest that oral anticoagulants should be considered (13).
Early coronary revascularization diminishes the risk of ischemic stroke with acute myocardial infarction. A delay in the acute revascularization of these patients influences the risk of perimyocardial infarction ischemic stroke independent of the infarction size or residual ventricular function. In a study, 45,997 patients who received thrombolytic therapy and 47,876 patients who were treated with primary percutaneous transluminal coronary angioplasty for myocardial infarction were stratified based on the time from presentation to initial therapy. A statistically significant linear relationship between time to revascularization therapy and risk of in-hospital ischemic stroke was seen on univariate analysis. Thrombolytic therapy within 15 minutes was associated with a lower risk of ischemic stroke (99).
Anticoagulation with full-dose heparin decreases the risk of left ventricular thrombi in patients with acute anterior myocardial infarction and may effectively reduce the risk of embolization in those with left ventricular thrombi. Aspirin reduced the risk of early ischemic stroke by half in the ISIS-2 mega-trial (05). Long-term oral anticoagulant treatment in survivors of myocardial infarction has been shown to reduce the frequency of stroke by 40% to 50% over a 3-year period (10; 56). Hemorrhagic complications are an uncommon but serious occurrence in patients treated with long-term oral anticoagulants, with rates related to the intensity of anticoagulation. After acute myocardial infarction, anticoagulation therapy is indicated for embolic stroke prevention, and antiplatelet therapy is a matter of ongoing investigation (30; 43). Statins reduce the risk of stroke among patients at risk for atherothrombotic disease (38), and preliminary data suggest they may reduce the risk of stroke during the early period after acute coronary syndrome (101).
In a study, among a cohort of elderly patients who survived an anterior myocardial infarction, there was no benefit from the use of warfarin up to 90 days post-myocardial infarction to prevent ischemic stroke (98). The authors performed a cohort analysis of 10,383 patients who survived hospitalization for an acute myocardial infarction. The primary outcome was 4-year ischemic stroke rates compared between anterior and non-anterior myocardial infarction patients. Within 4 years, 169 patients (5.7%) were admitted with an ischemic stroke. There was no significant difference in stroke rate between anterior and non-anterior myocardial infarction patients. The use of warfarin for up to 90 days was not associated with stroke protection after anterior myocardial infarction. The use of angiotensin-converting-enzyme inhibitors and beta blockers was associated with a significant decrease in stroke risk.
Risk factors for embolic stroke and intracranial hemorrhage following myocardial infarction differ. Patients at increased risk of embolic stroke, such as those with large anterior infarctions, may have the greatest survival benefit from thrombolytic therapy. The risk of intracerebral hemorrhage was reported to increase with the following: age; a history of hypertension, cerebrovascular disease, or head trauma; a high dose of thrombolytic therapy; being of female gender; being of African ancestry, having a low body weight; high blood pressure on presentation; and excessive prolongation of the aPTT with heparin (34; 37; 18; 45). Life-threatening ventricular arrhythmia and hypofibrinogenemia may play a role in some cases (86). The higher-than-anticipated rate of intracranial hemorrhage among patients treated with heparin or hirudin in conjunction with thrombolysis in several trials emphasizes the risks of more potent anticoagulation in combination with thrombolysis. The recommended heparin regimen is of a lower dose and is weight-adjusted (32). An analysis combining data from different thrombolytic trials identified four independent predictors for intracranial hemorrhage: (1) age over 65 years, (2) body weight below 70 kg, (3) hypertension on hospital admission, and (4) administration of alteplase (85). Models for individual risk assessment were developed that can be used early in clinical practice (85; 18). Although a history of stroke increases the overall risk of intracranial hemorrhage, preliminary observational data suggest that thrombolytic therapy may be beneficial in selected patients with acute myocardial infarction and a history of a nonrecent stroke (95). Such patients may be treated by primary coronary angioplasty, but the option of thrombolytic therapy should not be categorically excluded.
The development of stroke is one of the primary reasons for mortality after coronary artery bypass operations. It is essential to take all the measures to prevent this complication, especially in patients with known risk factors. The evaluation of carotid arteries before operation and the application of routine intraoperative epiaortic echocardiography may, in part, eliminate stroke (77). Preoperative Echo-Doppler study, performed in cases of coronary artery bypass grafting, followed by carotid endarterectomy under local anesthesia in patients with critical carotid stenosis, reduces the incidence of postoperative stroke (25).
Increased ticagrelor (a platelet aggregation inhibitor) usage in the management of acute myocardial infarction has led to a decreased incidence of ischemic stroke. In addition, percutaneous coronary intervention and statins were also associated with a decreased risk of ischemic stroke in patients with myocardial infarction (40).
Most strokes can be readily recognized. A patient with an acute myocardial infarction who develops a rapid change in neurologic status should be suspected of suffering a stroke. Rarely, other brain disorders (ie, seizure, tumor, subdural hematoma, or demyelination) and some metabolic abnormalities (ie, hypoglycemia or hypoxemia) may present in a similar fashion.
A combination of COVID-19-associated coagulopathy, extensive endothelial damage, and microvascular thrombosis frequently results in serious cardiac abnormalities, such as myocarditis, myocardial infarction, cardiomyopathy, and cardiac arrhythmias. A combination of hemodynamic alterations and COVID-19-associated coagulopathy enhances stroke risk as well (84).
The assessment of stroke complicating myocardial infarction should include substantial efforts to delineate the stroke subtype. Brain imaging (CT scan or MRI) is essential for distinguishing between infarct and hemorrhage. In trying to determine whether the etiology of a cerebral infarction following myocardial infarction is, indeed, of embolic origin or of another etiology (ie, large vessel or small vessel atherosclerotic disease), neurologic features are insufficiently predictive to establish the etiology. Echocardiography and other ancillary tests, such as duplex examination of the cervical arteries and transcranial Doppler, may help define specific vascular lesions and disease processes responsible for the stroke.
A high degree of suspicion is needed when caring for patients treated with a thrombolytic agent to establish a diagnosis as rapidly as possible. A decreased level of alertness or appearance of a neurologic deficit, particularly during the first 24 hours after thrombolytic infusion, should raise suspicion of an intracranial hemorrhage until proven otherwise (86). Thrombolytic, anticoagulant, or combined therapies should be discontinued as soon as symptoms and signs are recognized. Coagulation studies should be obtained immediately to document the severity of the coagulopathy, and preparations for reversing homeostatic abnormalities should be made, although the appropriate management is unclear (22). An emergent CT scan of the brain is necessary to distinguish intracranial hemorrhage from cerebral infarction and other neurologic disorders. It occasionally may be difficult to differentiate an intracerebral hemorrhage from a cerebral infarction with hemorrhagic transformation.
The patient with acute ischemic stroke requires appropriate supportive care and treatment of acute complications. Following an acute cardioembolic stroke due to left ventricular thrombi, risk for a recurrent early embolic event is high. To decide when to start anticoagulant treatment, one has to balance the benefit of reduction in early recurrent embolism against the risk of potentiating secondary brain hemorrhage. Cardioembolic strokes have a propensity for secondary hemorrhagic transformation. Large embolic infarcts, as well as infarcts visible on early CT scans and with mass effect, are especially prone to secondary hemorrhage. Most spontaneous hemorrhagic transformations occur within 2 to 4 days of cardioembolic stroke but are rarely associated with clinical deterioration. Conflicting evidence exists concerning whether anticoagulation during this period can result in neurologic impairment; therefore, no consensus has been reached on the optimum strategy.
In any central nervous system bleeding, early cooperation of cardiologists with neurologists, neurosurgeons, and hematologists will optimize management decisions. Immediate treatment with protamine (if the patient received heparin), cryoprecipitate, fresh frozen plasma, platelets, and an antifibrinolytic agent should be considered for an algorithm to manage bleeding complications (22). Although onset may be catastrophic and rapidly fatal, rapid neurosurgical intervention may be beneficial in selected cases, as suggested in the GUSTO I experience (60), but evidence from randomized clinical trials is lacking. Greater availability of primary angioplasty should decrease stroke rates, and the introduction of newer thrombolytic agents, weight-adjusted administration of heparin, low-molecular-weight heparins, and a new generation of antiplatelet drugs such as the glycoprotein IIb/IIIa receptor antagonists may also affect stroke rates as well as determinants of intracranial hemorrhage in patients with acute myocardial infarction (45).
Ticagrelor, a platelet aggregation inhibitor, reduces the risk of major adverse cardiovascular events when added to low-dose aspirin in patients with prior myocardial infarction. A randomized controlled study observed that in high-risk patients with prior myocardial infarction, the addition of ticagrelor 60 mg twice daily significantly reduced the risk of stroke (one-third of which are either fatal or lead to moderate-to-severe disability) without an excess of hemorrhagic stroke, but with more incidences of major bleeding (16; 39). In some preliminary reports, cangrelor infusion has shown promise. In two patients, cangrelor infusion was started during the percutaneous coronary intervention and continued for up to 48 hours in a dosage of 0.75 mcg/kg/min (72). None had ischemic or hemorrhagic complications during their hospital stay.
Patients suffering from both acute myocardial infarction and stroke are at an especially high anesthetic risk and, thus, are prone to high perioperative morbidity and mortality. Indeed, those who have suffered a recent myocardial infarction demonstrate high perioperative infarction and death. During neurosurgical procedures, the degree and potential for an increase in intracranial pressure are important considerations. The more information available to predict potential intraoperative and postoperative problems, the better the anesthesiologist can avoid or lessen the risk of those events.
All contributors' financial relationships have been reviewed and mitigated to ensure that this and every other article is free from commercial bias.

Ravindra Kumar Garg DM
Dr. Garg of King George's Medical University in Lucknow, India, has no relevant financial relationships to disclose.
See Profile
Steven R Levine MD
Dr. Levine of the SUNY Health Science Center at Brooklyn has no relevant financial relationships to disclose.
See ProfileNearly 3,000 illustrations, including video clips of neurologic disorders.
Every article is reviewed by our esteemed Editorial Board for accuracy and currency.
Full spectrum of neurology in 1,200 comprehensive articles.
Listen to MedLink on the go with Audio versions of each article.
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Stroke & Vascular Disorders
Feb. 08, 2025
Stroke & Vascular Disorders
Dec. 29, 2024
Stroke & Vascular Disorders
Oct. 29, 2024
Stroke & Vascular Disorders
Oct. 29, 2024
Stroke & Vascular Disorders
Oct. 29, 2024
Stroke & Vascular Disorders
Oct. 29, 2024
Stroke & Vascular Disorders
Oct. 29, 2024
Sleep Disorders
Oct. 14, 2024