Epilepsy & Seizures
Tonic status epilepticus
Jan. 20, 2025
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Worddefinition
At vero eos et accusamus et iusto odio dignissimos ducimus qui blanditiis praesentium voluptatum deleniti atque corrupti quos dolores et quas.
Visual-sensitive seizures elicited mainly by video games, television, and flickering lights in nightclubs are the more common reflex seizures, and most physicians can expect to encounter them. Children and teenagers are more vulnerable. However, in modern life with the increasing numbers of video game players and the spread of visual electronic media, the demographics of visual-induced seizures have expanded to nearly any age, and they sometimes get to epidemic proportions. This updated article reviews these seizures and their triggers and explains the basics of photosensitive epilepsy, pattern-sensitive epilepsy, fixation-off sensitivity, and seizures triggered by television screens, video games, computers, and cell phones. It details developments in their clinical manifestations, differential diagnosis, pathophysiology, and genetics as well as means of their detection and management. It emphasizes populations at risk, factors of misdiagnosis, treatment strategies, avoidance of various triggers, and certain antiepileptic drugs that may aggravate the seizures.
|
• Visual-sensitive seizures are provoked by photic, pattern, and other visual stimuli, alone or in combination. | |
|
• They are the commonest type of reflex seizures and are mainly triggered by video games, computers, cellphones, and flickering lights of nightclubs. | |
|
• Children and teenagers are more affected by visual-sensitive epilepsies, but people of all ages can be affected due to the widespread usage of visual media. | |
|
• Clinically, generalized seizures (absences, myoclonic jerks, generalized tonic-clonic seizures) are more common than focal seizures, which are usually visual. | |
|
• Certain epileptic syndromes (eg, juvenile myoclonic epilepsy, Dravet syndrome, Unverricht-Lundborg disease) commonly manifest with photosensitive seizures. | |
|
• The role of EEG is fundamental in identification of the offending stimuli with significant clinical and pathophysiological implications. | |
|
• Avoidance, prevention, or modification of the provocative triggers is the key point of management. |
Seizures triggered by visual stimuli were known in classical antiquity (123; 90; 57; 10; 71; 94; 63).
The first reference to photosensitive epilepsy is attributed to Apuleius Lucius (150 AD), a Roman philosopher in his “Apologia and Florida.” However, Apuleius does not refer to flickering lights:
|
Nay, even supposing I had thought it a great achievement to cast an epileptic into a fit, why should I use charms when, as I am told by writers on natural history, the burning of the stone named gagates is an equally sure and easy proof of the disease? For its scent is commonly used as a test of the soundness or infirmity of slaves even in the slave-market. Again, the spinning of a potter's wheel will easily infect a man suffering from this disease with its own giddiness. For the sight of its rotations weakens his already feeble mind, and the potter is far more effective than the magician for casting epileptics into convulsions (Apuleius Lucius, 150 AD). |
The oldest clear reference to photosensitive epilepsy is by Soranus Of Ephesus (2nd century AD) a Greek gynecologist, obstetrician, and pediatrician, who in Acute and Chronic Diseases, which contains an excellent chapter on nervous disorders, wrote:
|
The use of flame, or very bright light obtained from flame, has an agitating effect. In fact when a case of epilepsy is in its quiescent stage, the ultimate use of light with its sharp penetrating action may cause the recurrence of an attack. (Soranus Of Ephesus, 2nd century AD). |
Clementi was the first to describe experimental, light-induced epilepsy in studies of photic stimulation in dogs after strychnine application to the visual cortex (35). The effective triggering stimuli had to be repetitive. The following quote is from an English translation of Clementi’s report (71):
|
Under such experimental conditions, (continuous strychninisation of dog occipital cortex for 20’-30’), photic stimulation triggered an epileptic attack that began after a few minutes with nystagmus, mydriasis, and tonic eye deviation toward the side contralateral to the strychninised hemisphere, and continued with clonic movements involving first the periocular muscles and then the entire body. . . . Noteworthy extension of the strychninised occipital cortical area appears to be a necessary condition for onset of reflex epilepsy if strychninisation is limited to a single hemisphere. If, on the other hand, strychnine is applied over both hemispheres, strychninisation of a highly limited area may be sufficient (35). |
The first clinical evidence of photosensitive epilepsy by Gowers and later by Holmes refers to occipital seizures induced by light (67; 83).
|
In very rare instances the influence of light seems to excite a fit. I have met with two examples of this. One was a girl of seventeen whose first attack occurred on going into bright sunshine for the first time, after an attack of typhoid fever. The immediate warning of an attack was giddiness and rotation to the left. At any time an attack could be produced by going out suddenly into bright sunshine. If there was no sunshine an attack did not occur. | |
|
The other case was that of a man, the warning of whose fits was the appearance before the eyes of “bright blue lights, like stars--always the same.” The warning, and a fit, could be brought on at any time by looking at a bright light, even a bright fire. The relation is, in this case, intelligible, since the discharge apparently commenced in the visual centre (67). |
Holmes attributed this “reflex epilepsy” to an enhanced excitability of the visual cortex:
|
Some men subject to epileptiform attacks commencing with visual phenomena owing to gunshot wounds of the occipital region, have told me that bright lights, cinema exhibitions and other strong retinal stimuli tend to bring on attacks (83). |
Radovici and associates in 1932 reported the first case of eyelid myoclonia (often erroneously cited as self-induced epilepsy) with experimental provocation of seizures documented with cine film (150).
|
AA...age de 20 ans, presente des troubles moteurs sous forme de mouvements involontaires de la tete et des yeux sous l' influence des rayons solaires. |
Goodkind in 1936 also detailed various methods used to experimentally induce “myoclonic and epileptic attacks precipitated by bright light” in a photosensitive woman:
|
The patient was placed on a bed in a darkened room in such a position that when the black window shade was raised, her face only was directed towards the early afternoon sunlight, which came through an ordinary wire window screen. On such exposure of the eyes to the sun, she responded within a few seconds with marked, diffuse, and apparently uncontrollable clonic jactitatory movements. The movements ceased the moment a blindfold was applied or the black window shade was lowered. She reacted definitely also when either eye was uncovered separately. . . . The patient was also exposed to ultra violet radiation from a quartz mercury vapour lamp and to bright pocket flash light, to little or no effect. A small beam from a carbon arc lamp produced several rapid myoclonic jerks (66). |
With the advent of EEG by Berger in 1929, a new era started for the study of photosensitive epilepsies. Adrian and Matthews in 1934 were the first to introduce intermittent photic stimulation in the use of EEG (01). The subject was looking at an opal glass bowl that was illuminated from behind by a lamp, in front of which a disc with cut-out sectors was rotated.
Strauss in 1940 was the first scientist to record epileptic seizures induced experimentally by photic stimulation (168). His patient, a woman aged 33 years, had suffered right hemiparalysis and right Jacksonian fits from childhood. The epileptic attacks could be provoked by various sensory stimuli (tactile, auditory, visual etc.). He recommended that these stimuli should be noted not only by purely clinical observation but also, if possible, by EEG studies on the patient.
|
Flashing light into the right eye was associated with changes in the electroencephalogram. Three-per-second waves at high potential appeared, associated with twitching around the right corner of the mouth. The slow waves did not appear when the same stimulus was applied after cocainization of the right eye. The potentials, without a doubt, were true brain potentials because they could not be reproduced by having the patient imitate the twitching activity. Moreover, their appearance in the record from the left side makes it improbable that they represent muscle potentials from the muscles on the right side of the face (168). |
The real interest and detailed study of epilepsy by means of intermittent photic stimulation (IPS) activation was established by Walter and his associates in Bristol, England who started using a high intensity lamp of strobotron light to produce IPS (190; 191; 189). They found that IPS at a “magic frequency,” mostly 12 to 18 Hz, could induce subjective and objective symptoms, which correlated with specific EEG patterns. The most dramatic EEG abnormalities occurred in patients, mostly children, with a history of seizures but also to a lesser extent in subjects with only a family history of epilepsy. Clinically, these could be associated with bilateral or asymmetrical myoclonic jerks and “petit mal” attacks, rarely in combination.
Henri Gastaut and his associates in Marseilles, France have made numerous contributions to what we know about photosensitive epilepsies (60; 59; 58; 191; 61).
EEGs from the majority of patients with photosensitivity showed generalized discharges, and the view that photosensitivity was a generalized “centrencephalic” epilepsy dominated the relevant literature (60; 58; 15; 16).
Panayiotopoulos was the first to document that photosensitive epilepsy originates from the occipital regions (123).
Since then, different visual stimuli have been added to the list of visual-induced seizure possibilities. The problem of color was evident in 1997 when the Pokemon phenomenon occurred and was studied extensively (86; 174).
Numerous research groups, from basic science to the clinic, have been working on photosensitivity. They found that the physical characteristics of visual stimuli that can induce seizures include factors such as light intermittent frequency, intensity, contrast, type of color, and distance from the incentive. These characteristics are clues to trigger seizures, prevent them, and evaluate treatments (171).
The significance of reflex visual seizures lies in their connection to the external world and the brain, providing valuable insights into various aspects of epilepsy, including neural network mechanisms and treatment efficacy.
It is important to note that although visual sensitivity refers to an individual susceptibility to visual stimuli to trigger seizures, photosensitivity is a specific type of sensitivity that can trigger epileptiform activity on EEG in some individuals. Photoparoxysmal responses (PPR) were considered to be primarily generalized (58; 61; 12; 79), although the initial occipital onset of the generalized EEG discharge has been reported (137; 123; 123). Both visual sensitivity and photosensitivity are closely connected as they involve processing visual stimuli in the brain, and they have been extensively studied.
In 2012, a European group of experts led by Kasteleijn-Nolst Trenite emphasized the importance of testing photosensitivity on EEG with a correct and standardized activation protocol, promoting a European algorithm for visual stimulation in the EEG laboratory. This method is internationally recognized and is recommended as the appropriated method to test photosensitivity.
Modern functional image techniques, like EEG-MRI and PET, have increased the comprehension of the dysfunctional networks involved in the abnormal response to visual stimuli.
Nomenclature and classification. For a long time, the value of reflex seizures, like visually induced seizures, in epilepsy diagnosis was debated. In 2014, the International League Against Epilepsy’s (ILAE) practical clinical definition of epilepsy assumed that reflex seizure has the same value as an unprovoked seizure in defining epilepsy. (52).
The ILAE defines reflex seizures as seizures regularly induced by specific stimuli, such as sensory, sensory-motor, or cognitive, which cannot be avoided daily. Visual sensitivity is the most common form of stimulus-evoked seizure. Visual-sensitive epilepsies are enduring conditions predisposed to seizures induced by different physical characteristics of visual stimuli (153).
In 2022, the ILAE recognized two types of visually sensitive epilepsy syndromes: (1) photosensitive occipital lobe epilepsy (POLE) and (2) epilepsy with eyelid myoclonia. Although other visual epilepsies are not considered independent syndromes, seizures induced by visual stimuli can occur in syndromes such as juvenile myoclonic epilepsy, other generalized genetic epilepsies (GGE), developmental and epileptic encephalopathies (DEE), and other syndromes, such as Dravet syndrome.
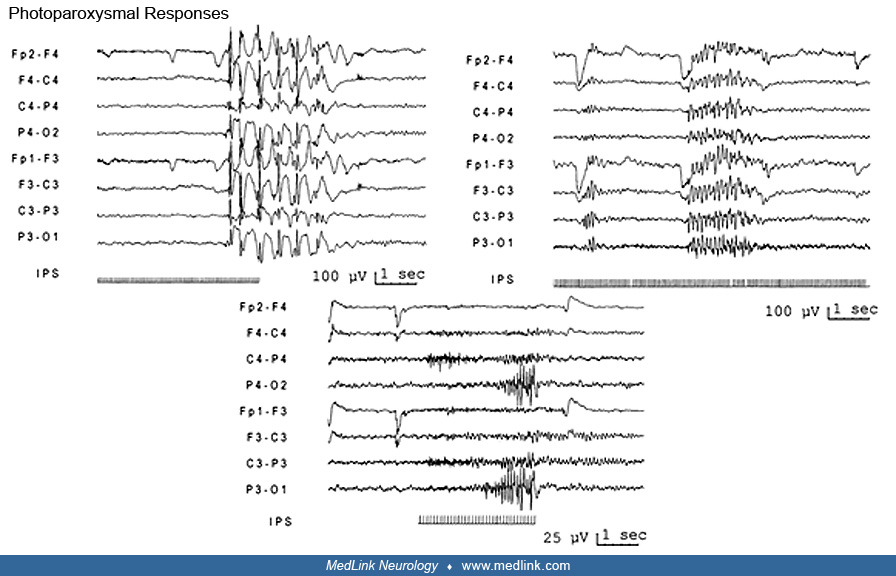
Photosensitivity is the abnormal response to intermittent photic stimulation on EEG termed “photoparoxysmal response” (PPR). It consists of epileptiform discharges (spikes, polyspikes, or spike-and-wave complex) that are more frequently bilateral and more or less extended. Bilateral epileptiform discharges are the most frequent responses (58; 61; 12; 79), but they can occur with initial occipital onset (137; 123; 123).
Intermittent photic stimulation (IPS) responses were initially classified by Waltz and colleagues (192). Kasteleijn-Nolst Trenite and colleagues proposed an ILAE classification system for IPS responses in 2001. A simplified version by Meritam Larsen has been proposed to improve clinical utility and interobserver agreement (115).
Visual-induced seizures are the commonest type of reflex seizures. They are triggered by the physical characteristics of certain visual stimuli and not by their cognitive effects. Photosensitivity and pattern sensitivity are the two main categories (with frequent overlap) of simple reflex seizures with short (typically within seconds) time between stimulus and response.
According to the predominant visual stimuli, patients with visual-sensitive epileptic seizures can be grossly divided as:
|
• photosensitive |
Visual-sensitive epilepsies are the enduring conditions that cause seizures triggered by various visual stimuli. Visually triggered seizures are the most common type of reflex seizures (201; 07; 171). They are induced by the physical nature of the stimuli, not their cognitive effects.
There are two main types of visually sensitive epilepsies depending on the characteristics of stimuli that trigger them. The first type is called photosensitive epilepsy, which is related to the frequency, intensity, and color of light stimuli. The second type is called pattern-sensitive epilepsy, which depends on the orientation of lines, contrast, and patterns and the frequency of changes. Additionally, the absence of visual stimulation, known as fixation-off, is also considered part of this group.
Visual-sensitive epilepsies include various clinical situations and epileptic syndromes that share similar clinical characteristics, such as age of onset, seizure type, EEG findings, and treatment response (Table 1). People who are prone to seizures triggered by contemporary visual stimuli may suffer from video game–sensitive epilepsy, TV-sensitive epilepsy, or similar conditions caused by computers and cell phones. However, these individuals do not differ from the clinical perspective of epilepsy except for the stimuli that trigger the seizure (134; 122).
Although visual-sensitive epilepsies are associated with generalized epilepsies (58; 61; 12; 79), there are also focal epilepsies, most of which have photosensitivity as a common background (137; 123; 123; 201). The etiology is frequently genetic, but structural epilepsies have also been described.
|
Type of epilepsy |
Generalized |
Focal |
|
Type of seizure |
Myoclonic, myoclonic tonic-clonic, bilateral tonic-clonic, absences |
Visual seizures |
|
Age of onset |
Variable from infancy to early adulthood (8–14 years old) |
Variable from 1 to 50 years (4–17 years old) |
|
Trigger stimuli |
Flickering lights, patterns, video games, TV, movies, Internet and social media, LEDs |
Flickering lights, patterns, video games, TV, movies, Internet and social media, LEDs |
|
EEG findings |
Bilateral spikes, polyspikes, or spike-and-wave complex |
Occipital spikes |
|
IRS response |
Bilateral spikes, polyspikes, or spike-and-wave complex |
Occipital spikes or bilateral spikes |
|
Syndromes |
epilepsy with eyelid myoclonia, juvenile myoclonic epilepsy |
POLE |
|
Etiologies |
Genetic |
Genetic |
|
Sex |
Female>Male |
Female>Male |
Photosensitive epilepsy. Photosensitive epilepsy is a broad term comprising numerous heterogeneous situations in which seizures are triggered by light (79; 205; 175; 132; 73; 134; 122). It is not a syndrome on its own but rather a part of different syndromes and clinical conditions.
The presence of photosensitivity can be measured objectively with EEG by the presence of an abnormal response, hotoparoxysmal responses, during photic stimulation. The prevalence of photosensitivity has been extensively studied. It can be present in healthy individuals at a range of 1.4% to 11.6% depending on geographic location, sex, age, and photic stimulation protocol used. Studies show that photoparoxysmal responses are more frequent in females aged 7 to 12 years (151; 160).
The role of photosensitivity in epilepsy is controversial, and it was found to be related to spontaneous epileptiform discharges in the EEG and family history of epilepsy. Kasteleijn-Nolst Trenite reported that up to 63% of photosensitive patients had a history of clinical seizures when questioned in detail (93).
Photosensitivity is a common occurrence in GGE, especially in idiopathic generalized epilepsies such as juvenile myoclonic epilepsy, where it affects 30% to 90% of patients. It occurs less frequently in other idiopathic generalized epilepsies, such as childhood (18%) and juvenile (8%) absence epilepsies, and epilepsy with generalized tonic-clonic seizures alone (13%). Other GGE, such as myoclonic epilepsy in infancy (207), or syndromes like Dravet syndrome, Lennox-Gastaut syndrome, Lafora disease, Unverricht-Lundborg disease, or myoclonic epilepsy with ragged-red fibers, can also exhibit photosensitivity as a frequent symptom (202; 154; 06; 79; 193; 72; 167; 112; 53).
Pure photosensitive epilepsy is a rare condition that causes reflex seizures triggered by visual stimuli, without the occurrence of spontaneous seizures. It was first described by Harding and Jeavons in 1994 and later by Covanis in 2005 (37). These forms of visually sensitive epilepsies are not commonly observed and are often associated with noninduced seizures. They are not recognized as an independent syndrome.
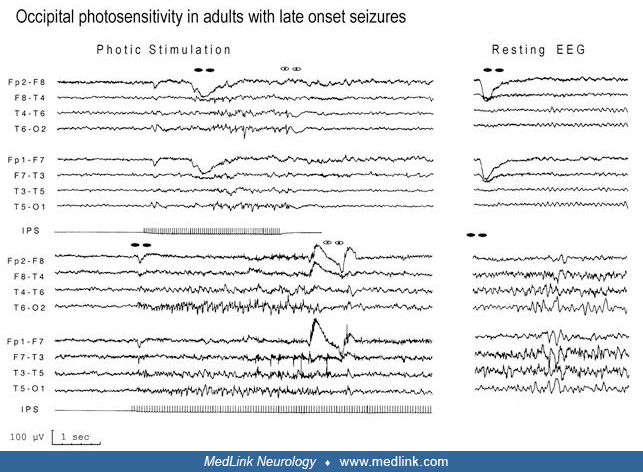
Photosensitive epilepsy can occur in childhood focal occipital lobe epilepsies (POLE) (166), but Koutroumanidis reported a group of adults with focal photosensitive epilepsy in the occipital lobe (106).
Precipitants of seizures and environmental stimuli. The sources that can trigger seizures are numerous and diverse, ranging from sunlight filtering through trees to the rotating blades of a helicopter. Moreover, the risk of seizures caused by visual stimuli has increased due to the proliferation of artificial sources, such as stroboscopic lights, televisions, video games, computer screens, cell phones, and flashing lights in nightclubs (171). Stimuli that flicker at medium- to high-range frequencies and have high contrast, high luminance, and color modulation are the most likely to cause seizures. Various forms of media, such as video games, TV shows, movies, the Internet and social media, LEDs, and 3D technology, can trigger seizures (182; 53). It has been reported that seizures can be caused by seemingly harmless activities, such as playing mahjong (04) or taking a selfie (26). As a result, more research is necessary to identify potential triggers of seizures and patients should take necessary precautions to avoid them.
Various factors, such as sleep deprivation, alcohol and drug consumption, and emotional and physical stress, can increase the risk of seizure induction by flickering lights. Adolescents who attend electronic dance music concerts are particularly susceptible to such risks (156).
Photically induced seizures. Generalized seizures (absences, myoclonic jerks, and GTCS) are more frequent than focal photically induced seizures.
Generalized seizures. Generalized tonic-clonic seizures are the most reported (55% to 84%), followed by myoclonic or myoclonic tonic-clonic seizures. Absences and absences with eyelid myoclonia are less frequently reported (6% to 20%) (79; 202; 201). Subtle seizures may go unnoticed due to the way patients are questioned about their medical history. Studies have shown that about half of the patients who experience seizures during video-EEG monitoring do not report them when asked (93; 23). This may be because patients may not be aware of these subtle seizures, which can include different types, such as eyelid myoclonias, absence seizures, or a combination of them. As a result, it is important for healthcare providers to be aware of these less noticeable seizures and to ask specific questions to help identify them.
Focal seizures. Focal seizures are less commonly reported and may be followed by bilateral tonic-clonic seizures in patients with occipital lobe epilepsy. Focal seizures arising from regions other than the occipital lobe are exceptional (13; 201; 53).
Pattern-sensitive epilepsy. Pattern-sensitive epilepsy refers to epileptic seizures induced by patterns, typically stripes (79; 195; 19; 148; 149; 25). It is not a particular epileptic syndrome, and it is closely related to photosensitivity. Almost all patients with clinical pattern sensitivity epilepsy show photoparoxysmal responses. The sensitivity to pattern depends on the characteristics of the stimuli, such as contrast, width, and direction of lines. Vertical lines are more potent than horizontal or diagonal ones (171). Most patients with this condition exhibit photosensitivity and a PPR during intermittent photic stimulation on EEG. Meanwhile, 30% of patients with photosensitive seizures are sensitive to appropriate patterns. When different patterns are combined with intermittent light, the photic stimulation effect is amplified (91).
Patients with visually sensitive epilepsies are at a high risk of experiencing pattern sensitivity, and this likelihood can differ based on their age and the duration of stimulation. Unlike in photosensitive patients, there is no significant difference in the prevalence of this condition between males and females (160). According to Kasteleijn-Nolst Trenite's findings, 50% of patients with PPR during photic stimulation experienced pattern sensitivity.
The symptoms of pattern sensitivity are similar to those seen in photosensitive epilepsy. Some studies have found that absence seizures and occipital seizures occur more frequently than myoclonic or tonic-clonic seizures (93). A systematic review by Radhakrishnan in 2005 found that generalized tonic-clonic seizures were the most common type of seizure. Other studies, such as the one conducted by Brinciotti and colleagues, found that focal seizures were the most common reflex seizure type in pattern-sensitive patients (25).
Pattern sensitivity without photosensitivity, sensitivity to nongeometric patterns, and self-induced pattern-sensitive epilepsy are rare.
Environmental stimuli. Environmental stimuli that induce seizures in pattern-sensitive patients are those that best match the properties of the provocative patterns used in relevant EEG testing and best suit and create the conditions of their spatial and directional presentation to the eyes. These are striped clothes (eg, shirts, jackets, or ties), escalators, wallpaper, furnishings, venetian blinds, air conditioning grills, and radiators. Any activity visually involved with these patterns, such as ironing, is likely to induce seizures. Less direct, but often very significant, is the role of patterns in more complex stimuli, such as TV viewing and video games (79; 195; 19).
Pattern is recognized as a seizure precipitant less often by patients, caregivers, and physicians than environmental flicker and specific agents, such as the TV, nightclub lighting, or video games. Direct questioning implicates pattern as a seizure trigger in 6% to 30% of photosensitive individuals (200; 93).
Modern life visually provoked seizures. It has been known since 1980 that video games can trigger seizures, especially among young boys. In Great Britain, Quirk found an incidence of 1.5 cases per 100,000 first seizures induced by video games in individuals aged between 7 to 19 years old (146). Studies have also shown that TV screens can cause visual seizures, with factors such as the color of the screen, screen size, frequency, and brightness being important contributors. However, the advent of digital screens has reduced the likelihood of this phenomenon happening again.
Despite this, the growing use of portable devices, cell phones, and the Internet and social media has exposed millions of people to stimuli that can still trigger seizures. In a report for the Epilepsy Foundation, Fisher advised caution regarding the use of videos as they can be made using animation without supervision and even with malicious intent to induce seizures, as has already been reported (53).
Limited information is available regarding the potential impact of other sources of stimulation, such as 3-dimensional movies, video games, as well as virtual reality. Although some reports suggest that there is no increased risk (182), it is important to note that this is heavily dependent on technical aspects.
From a clinical perspective, visually sensitive seizures can take various forms, including absence seizures, myoclonic jerks, and bilateral tonic-clonic seizures; focal seizures have also been documented.
The factors that contribute to seizures are a complex combination of photic, pattern, and cognitive stimulation. Furthermore, other factors, such as sleep deprivation, emotional stress, and fatigue, can increase seizure induction chances.
Based on a report by Kasteleijn-Nolst Trenite, 85% of patients were found to be sensitive to IPS, whereas 40% of the same group were sensitive to pattern stimulation (93). Other factors, such as the type of stimulation technique used during the EEG recording, have also been found to affect the percentage of sensitivity reported in previous studies (131).
Video-induced seizures. The term “video-induced seizures” is preferable to “video game epilepsy” because this is not a syndrome. There is heterogeneity in seizure types, syndromes, precipitating and facilitating factors, and underlying mechanisms.
Video games comprise a multibillion-dollar industry with the demographics of players extending in nearly all ages and non-sex difference.
Video-induced seizures refers to epileptic seizures precipitated by playing interactive computerized “video games,” a term used to include not only those games using an interlaced video monitor but also small, hand-held, liquid crystal displays, arcade games, smart phones, and other new electronic devices that use noninterlaced displays and higher refresh rates than television (18; 50; 51; 68; 99). With video games pervading life today, the number of patients continues to rise. The risk has been highlighted by media reports. Manufacturers now warn of the danger and have sponsored research into the subject. The results of these studies prior to 1999, mainly sponsored by Nintendo Corporation under the auspices of the Japanese Epilepsy Association, were published in Epilepsia (55; 96; 116; 141; 152).
Clinical aspects. There are many mechanisms by which video games may induce seizures. These are photosensitivity, pattern sensitivity, emotional and cognitive excitation, and proprioceptive stimulation (movement). All these factors, alone or in combination, may induce a seizure. Fatigue, sleep deprivation, and hunger are significant contributing or facilitating factors (18; 50; 51; 68).
Risk of video-induced seizures in non-photosensitive patients with epilepsy. Millet and colleagues examined systematically whether exposure to video game material is a risk factor for seizures in patients with chronic epilepsy without visual sensitivity (116). Of 212 epilepsy patients without EEG photosensitivity, 13 had seizures during periods of video game play and 12 during alternative leisure. The authors concluded that seizures during video game play in more than 95% of the epilepsy population without visual sensitivity are most likely to represent a chance occurrence, although, as always, each individual should be carefully assessed.
Television-induced seizures. TV-induced seizures were the most common form of photosensitive epilepsy before the introduction of video games. The advent of digital screens has reduced the potential risk of seizure induction.
TV-induced seizures mainly affect children aged 10 to 12 years. There is a two-fold preponderance of girls. Seizures are more likely to occur when the patient is watching a faulty (ie, flickering) TV set or is sitting very near to the TV screen (less than 1 meter distance).
Screen content may be more important than the characteristics of the screen itself (27). Programs with flickering lights are particularly dangerous, and occasionally their effect on eliciting seizures may reach epidemic proportions. TV-induced seizures came to global public attention in December 1997 when approximately 700 children and adolescents in Japan were admitted to the hospital because of seizures provoked by a Pokémon (Pocket Monster) television cartoon (85; 174). The offending sequence was caused by a character’s actions in a rocket launch scene with a flashing red then blue screen, changing 12.5 times per second for 4 seconds.
Self-induced seizures. Patients with all types of visually induced seizures may willingly induce attacks to themselves. Maneuvers for self-induction aim to provoke a seizure by producing stimulation by flickering light, patterns, proprioceptive stimuli, or higher brain functions. Intensely pleasurable sensations have been reported with these types of seizures, and some patients induce seizures to relieve stress or to gain attention (177).
Demographics. The exact prevalence of self-induced seizures is difficult to determine and may have been overestimated. In many cases, “self-induced” behaviors do not appear to be willful or consciously generated, and eyelid blinking or forced eyelid deviation towards the light has been unquestionably taken as evidence of self-induction. Of 442 patients with onset of nonfebrile seizures from birth to 15 years of age, 5 (1.1%) had self-induced seizures (130). Age of onset varies from infancy to mainly early childhood, and females (70% to 80%) predominate.
The objective of self-induced seizures is relief of tension and anxiety or escape from a disturbing situation.
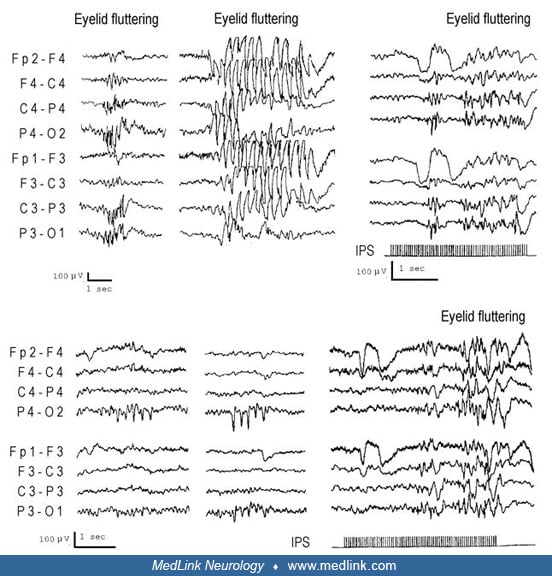
One maneuver for self-induction in photosensitive epilepsy is looking at a bright light source, usually the sun, and voluntarily waving the abducted fingers in front of the eyes (sunflower syndrome) in order to produce optimal intermittent photic stimulation. Other techniques are repetitive opening and closing of the eyes or lateral or vertical rhythmic movements of the head in front of a bright light source; making the television picture roll; quickly changing television channels while watching from a close distance; and playing video games.
A controversial aspect of self-induction is whether slow and forcible eye-closure is another maneuver used by photosensitive patients (40; 17) or part of the seizure (136). (See Clinical vignette 1.)
Differential diagnosis. Self-induced maneuvers should be differentiated from tics as well as genuine ictal phenomena, such as eyelid myoclonia or eye closures of occipital seizures (136).
Early forced eyelid blinking and flutter, eyelid jerks, and oculoclonic activity may be ictal manifestations of the occipital lobes that may not show in surface EEG but can be documented with deep stereo-EEG recordings.
Eyelid blinking and gaze fixation to light may be a normal “attraction movement” when light is presented and other manifestations of the optic fixation reflexes when volitional movements of the eyes are unattainable or weak.
Blinking functions may be a complex indicator of phasic responses to stress, such as that produced by listening to emotionally laden words.
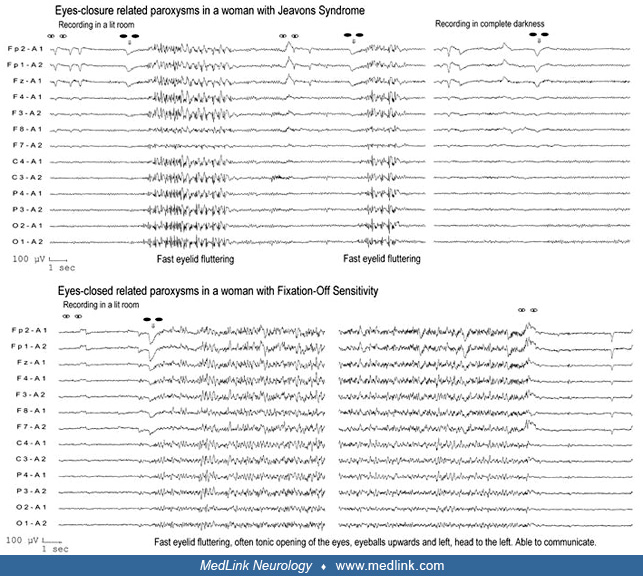
Fixation-off sensitivity. Fixation-off sensitivity (FOS) is a term coined by Panayiotopoulos to denote the form(s) of seizures or EEG abnormalities that are elicited by elimination of central vision and fixation (126; 127; 132). “Elimination of central vision and fixation”’ is a specific precipitating stimulus, which, even in the presence of light, induces high-amplitude occipital or generalized paroxysmal discharges. (See Clinical vignette 2.)
Fixation-off sensitivity can occur in patients with different syndromes, like POLE or childhood occipital visual epilepsy (COVE). However, it is not a definitive sign of these conditions. The model examples of fixation-off sensitivity are more frequent in patients with COVE than POLE (134; 89). Fixation-off sensitivity abnormalities are mainly localized in the occipital regions and are not associated with overt ictal clinical manifestations.
Patients are not photosensitive and differ markedly from those with Jeavons syndrome (eyelid myoclonia with absences).
However, fixation-off sensitivity has also been described in patients with structural epilepsy (109; 108; 49; 80; Saadeldin and Al-Tala 2011; 22; 48) and in a child with CHD2 mutation and mild developmental impairment (32). Furthermore, fixation-off sensitivity may occur in individuals without seizures (105; 107). In such an asymptomatic adult with fixation-off sensitivity, continuous bilateral occipital paroxysms during elimination of central vision were associated with transitory cognitive impairment, demonstrated by neuropsychological testing (107).
Fixation-off sensitivity has the opposite characteristics of photosensitive epilepsies (Table 2), but conversion from one to the other may rarely occur (125).
|
Fixation-off sensitivity epilepsy |
Photosensitivity epilepsy | |
|
Resting EEG in a lit recording room |
Eye-closed abnormalities |
Eye-closure abnormalities |
|
Effect of darkness |
Activation of abnormalities |
Inhibition of abnormalities |
|
Effect of fixation and central vision |
Inhibition of abnormalities |
Activation of abnormalities |
|
Effect of patterns |
Inhibition of abnormalities |
Activation of abnormalities |
|
Effect of IPS |
None or inhibition |
PPR |
|
| ||
Fixation-off sensitivity paroxysms studied with functional MRI were correlated with activation of parieto-occipital and frontal brain areas (107) and a significant increase of blood oxygen level-dependent signal in the extrastriate cortex (84). Magnetoencephalography of visual-evoked fields in fixation-off sensitivity revealed abnormal activation of the visual corticocortical pathway via the insular cortex (108). Strigaro and colleagues studied cortical excitability changes associated with fixation-off sensitivity in a woman with generalized fixation-off sensitivity (169). They measured by transcranial magnetic stimulation (TMS) the excitability level of the primary motor area and explored her visual system by pattern-reversal and flash visual-evoked potentials. They found that both outside and within fixation-off sensitivity the cortical silent period was dramatically short, indicating defective GABA-B inhibition as a persistent background factor. The same was true for the short-interval intracortical inhibition, a TMS marker of cortical GABA-A inhibition. The fixation-off sensitivity state corresponded then to a pathologic enhancement of intracortical facilitation, a TMS marker of Glu/Asp transmission. During fixation-off sensitivity, the flash visual-evoked potentials exhibited a hugely enhanced after discharge, expressing a pathologic overactivity of secondary visual areas. The authors concluded that these findings support a grossly imbalanced cortical excitability, in both the frontal and posterior areas, as an important correlate of fixation-off sensitivity.
Scotosensitive epilepsy. Scotosensitivity (skotos in Greek means darkness) denotes forms of epilepsy, seizures, or EEG abnormalities that are elicited by the complete elimination of retinal stimulation by light. Pure scotosensitive patients are rare (11). Most patients described as scotosensitive probably have fixation-off sensitivity (127).
Techniques for documenting fixation-off sensitivity (127; 134; 103; 104). First, it is essential to confirm that the EEG abnormalities observed in routine EEG recording are related to the eyes-closed state.
The patient is asked to open and close his or her eyes every 5 seconds, six times consecutively. Instructing the patient to look at a fixed point, such as the tip of a pencil, ensures fixation in the eyes-opened state (127).
Then, fixation-off sensitivity is evaluated by instructing the patient to perform the same sequence of eyes-opened and eyes-closed states in conditions that eliminate central vision and fixation. There are many practical ways to achieve this, such as underwater goggles covered with opaque tape (this achieves complete darkness) or semitransparent tape (which allows light in but obscures any other visual input).
Warning. Complete darkness can be difficult to achieve in routine EEG departments. Even a small spot of red light on which the eyes may fixate can totally inhibit EEG abnormalities induced by complete darkness. Switching off the lights in the EEG recording room is not adequate and may explain conflicting results in the literature. Complete darkness can be produced with underwater goggles covered completely with opaque tape (127).
Visually sensitive epilepsies have a prognosis that is related to the underlying condition of the seizures. There is a wide range of conditions, some of which will resolve with age, whereas others may lead to severe developmental encephalopathies. The general prognosis of remission of visually sensitive seizures is estimated to be between 14% to 37% according to Fisher and Harding (53). However, Brinciotti found a good prognosis for seizure control in patients with pattern-sensitive seizures, with 80% of patients remaining seizure-free in a follow-up period of more than 5 years (25).
In certain syndromes, such as epilepsy with eyelid myoclonia, it has been found that over 25% of patients suffer from drug-resistant epilepsy. This resistance to medication is linked to various factors, including comorbidities, such as psychiatric and intellectual disability, as well as photosensitivity and eye closure sensitivity (162).
The prognosis for POLE varies; some patients experience remission or only a few seizures, and others may continue to suffer from visually induced seizures (166). In a long-term follow-up study, it was found that 80% of patients achieved remission, but some experienced relapses and continued to exhibit photosensitivity (161). Despite these findings, Fisher has emphasized the need for further studies on the long-term follow-up of visually sensitive epilepsies, both in general and in specific syndromes (53).
Seizures that are triggered by visual stimuli can lead to complications similar to spontaneous seizures. There is also an increased risk of sudden unexpected death in epilepsy, especially in cases of generalized tonic-clonic seizures. Therefore, avoiding trigger stimuli is an important aspect of treatment.
Apart from seizure control concerns, the social outcomes of epilepsy patients are also significant. Despite having seizure control and normal intellectual functioning, children with epilepsy face higher rates of incomplete education, unemployment, poverty, social isolation, and psychiatric disorders in their adult lives (28). However, this finding is controversial, and Schneider-von Podewils showed that early control of seizures in patients with juvenile myoclonic epilepsy improves their long-term social outcomes (157).
Vignette 1. At the age of 5 years, this now 39-year-old woman experienced onset of frequent seizures manifested with brief but marked eyelid myoclonia and absences with mild or moderate impairment of consciousness. Absences improved, but eyelid myoclonia continued daily with ethosuximide treatment. At the age of 26 years, an attempt by a neurologist to substitute ethosuximide with carbamazepine resulted in nonconvulsive status epilepticus. With continuous eyelid myoclonia and absences, she became confused: “My eyes were continuously jerking. I was unable to look after myself and was drowsy and off work for a few days.”
She suffered a total of six GTCS throughout her life, starting at the age of 13 years. Two GTCS were induced by lights, and the others occurred after sleep deprivation, alcohol indulgence, or inappropriate change of medication. Eyelid myoclonia and GTCS occurred mainly in the morning after awakening. Seizures improved dramatically when valproate was added to ethosuximide at the age of 31 years, but she continued to have brief seizures of eyelid myoclonia without absences or GTCS. Ethosuximide was later replaced by clonazepam 0.5 mg nightly, which controlled the eyelid myoclonia. At 39 years of age, she has been seizure-free for 4 years on clonazepam 0.5 mg and valproate 2000 mg daily.
Over the years, a number of experts suggested that she was self-inducing her seizures. I have frequently questioned her regarding self-induction, which she thoroughly denies. “Why?” she said “It gives me no pleasure and it is socially embarrassing.” Similar clinical and EEG seizures also occurred while the eyes were closed as well as that the onset of the generalized discharges preceded the eyelid myoclonic jerks.
Vignette 2. This normal boy with COVE had, from the age of 10 years, experienced the following:
(1) Ictus amauroticus--brief, infrequent episodes of complete blindness without warning or impairment of consciousness.
(2) Visual seizures--frequent (1 to 3 per week), transient visual disturbances lasting for 10 to 30 seconds.
It looked like a rectangle filled with colored small circles. This time I saw the colors. They were blue, green, red and yellow. While I was reading, I started seeing the words all stuck together. I blink a lot to see more clearly. It is a familiar vision, sometimes bright or dark. It replaces, obscures the real images. They are large objects, probably people, which I cannot identify. They are always in my right eye and draws my right eye and my head to the right.
(3) Syncope-like epileptic seizures--four brief (1 to 2 minutes) episodes of loss of consciousness without convulsions while sitting or standing. He falls down and becomes unresponsive, but there are no postictal symptoms. One episode witnessed by a physician who described it as, “clumsiness, vacant, unresponsive for a minute or so. No convulsions.” Another seizure was witnessed by his parents: “he was next to us in a shop. We heard a bang and saw him on the ground. Color white not blue. He was out for a few seconds.”
No further episodes occurred after initiation of treatment with carbamazepine, which was stopped 3 years later. At last follow-up at 18 years of age, he was well and a good student; he was taking no treatment and had normal EEG and brain MRI.
Recent advances in neurophysiology, neuroimaging, and genetics have significantly expanded our understanding of visually sensitive epilepsies. Despite clinical and experimental evidence in animal models, the underlying mechanisms behind photosensitivity, pattern sensitivity, and visually induced seizures are not fully comprehended (122).
Genetics. The etiology of visually sensitive epilepsies appears to be predominantly genetic, with a high incidence of associated genetic epileptic syndromes. Our understanding of the genetics of photosensitivity is primarily based on the genetics of PPR, though the specific genes involved remain unclear.
A single gene for photosensitivity has not yet been identified, and the genetics of human photosensitivity probably involve several genes in different chromosomes. However, autosomal dominant inheritance with reduced penetrance was proven in several families with photosensitivity (87).
A family with a novel autosomal dominant familial epilepsy syndrome "myoclonic occipital photosensitive epilepsy with dystonia" has been described (155). This involves a spectrum of phenotypes from juvenile myoclonic epilepsy, sometimes overlapping with POLE to progressive myoclonus epilepsy with paroxysmal dystonia.
Photoparoxysmal responses. Although initial family studies suggest that the inheritance pattern of this trait is autosomal dominant, newer research has shown that it is more complex and influenced by multiple genes, as well as age, sex, and ethnicity. Caucasians have a higher prevalence of this trait compared to Asians and Africans (165). Reports of monozygotic twins show complete concordance in photosensitivity, and if the parents are photosensitive, up to 50% of their children may also have this genetic trait (47).
Although this trait is often associated with epilepsy, it is not always present and can be independently inherited in many syndromes.
Molecular genetic studies on EEG photoparoxysmal response identified putative loci on chromosomes 6, 7, 13, and 16 that seem to correlate with peculiar seizure phenotype (87).
Genetics of photoparoxysmal responses in epileptic syndromes. It is well documented that photoparoxysmal responses (PPR) with or without clinical photosensitivity are associated with greatly variable types of epilepsy and mainly with idiopathic generalized epilepsy (eg, juvenile myoclonic epilepsy), genetic epileptic syndromes (eg, Dravet syndrome), and a number of autosomal-recessive progressive myoclonic epilepsies (167). Therefore, the molecular genetics of photoparoxysmal responses may also vary.
Pinto and colleagues collected 16 photoparoxysmal response-multiplex families of mainly idiopathic generalized epilepsy with myoclonic jerks and conducted a genome-wide linkage scan using a broad model (all response types to IPS) and a narrow model (exclusion of response types I-II and the occipital epilepsy cases) of photoparoxysmal responses (143). They found empirical genome-wide significance for linkage for two chromosomal regions, 7q32 at D7S1804 and 16p13 at D16S3395, respectively. These genomic regions contain genes that could be important for the neuromodulation of cortical dynamics in humans, such as the genes encoding the metabotropic glutamate receptor 8 (GRM8) and the cholinergic-muscarinic type 2 acetylcholine receptor M2 (CHRM2) (139).
To explore genetic relations between photoparoxysmal responses and idiopathic generalized epilepsy, Tauer and colleagues studied 60 families with at least two siblings displaying photoparoxysmal responses; 19 families with predominantly pure photoparoxysmal responses and photosensitive seizures (PPR-families) and 25 families in which photoparoxysmal response was strongly associated with idiopathic generalized epilepsy (PPR/IGE-families) (178). They found two PPR-related susceptibility loci, depending on the familial background of idiopathic generalized epilepsy. The locus on 6p21.2 seemed to predispose to PPR itself, whereas the locus on 13q31.3 also confers susceptibility to idiopathic generalized epilepsy. In particular, MOD score analyses provided significant evidence for linkage to the region 6p21.2 in the PPR families (empirical p = 0.00004) and suggestive evidence for linkage to the region 13q31.3 in the PPR/IGE families (p = 0.00015), both with a best-fitting recessive mode of inheritance. In the PPR/IGE families, linkage evidence was even stronger (p = 0.00003) when the trait definition was broadened by idiopathic generalized epilepsy traits. However, an analysis of 100 families has not replicated these findings (42).
De Kovel and associates performed a whole-genome linkage scan for epilepsy-related photosensitivity (42). They combined two previously published genome-wide linkage studies (which have loci for photoparoxysmal responses at 6p21, 7q32, 13q13, 13q31, and 16p13) augmented with additional families in a mega-analysis of 100 families. Nonparametric linkage analysis identified three suggestive peaks for photosensitivity, two of which were novel (5q35.3 and 8q21.13), and one has been found before (16p13.3). No evidence for linkage was detected at 6p21, 7q32, 13q13, and 13q31. The authors concluded that different family data sets are not linked to a shared locus. Detailed analysis showed that the peak at 16p13 was mainly supported by a single subset of families whereas the peaks at 5q35 and 8q21 had weak support from multiple subsets.
Dibbens and associates screened NEDD4-2 (Neuronally Expressed Developmentally Downregulated 4) for mutations in a cohort of 253 families with idiopathic generalized epilepsy (43). They identified three NEDD4-2 missense changes in highly conserved residues, S233L, E271A, and H515P in families with photosensitive generalized epilepsy, and concluded that photosensitive epilepsy may arise from defective interaction of NEDD4-2 with as yet unidentified accessory or target proteins. This gene is involved in encoding a ubiquitin protein, and it has a role in the regulation of voltage-gated sodium channels, receptors, and transporters in the cell surface. These channel genes are involved in regulated neuronal excitability. Also, pathogenic variants of the SYNGAP1 gene were found in patients with DEE and visual sensitivity to fixation-off and eye closure (111).
A trend toward association of transient receptor potential cation 4 (TRPC4) variants and photoparoxysmal responses of idiopathic generalized epilepsy has been reported (187).
Taylor and colleagues used family studies to investigate the clinical genetics of photosensitivity to understand the interrelationship of different photosensitive epilepsy syndromes (179). Twenty-nine families were recruited in which at least two members had idiopathic epilepsy and either clinical or electrical photosensitivity on EEG studies. The authors performed electroclinical analysis of these individuals and all other affected family members and analyzed the phenotypic patterns in families. They found an earlier age at seizure onset in photosensitive patients compared with nonphotosensitive individuals. A significant female bias for photosensitivity was confirmed. All subjects with visual seizures were photosensitive. Subjects could be classified into three main photosensitive phenotypes: genetic (idiopathic) generalized epilepsies (GGE), idiopathic photosensitive occipital epilepsy (IPOE), and mixed GGE/IPOE. Within each category, subjects with purely photosensitive seizures were observed. The authors also reported a distinctive syndrome of early-onset photosensitive absence epilepsy, with onset beginning by 4 years of age, which was more refractory than childhood absence epilepsy. It was concluded that the clinical genetics of the idiopathic photosensitive epilepsies show a phenotypic spectrum from the GGEs to IPOE with overlap between the focal features of IPOE and all the GGE syndromes. Shared genetic determinants are likely to contribute to the complex inheritance pattern of photosensitivity, IPOE, and the GGEs (179).
CHD2 has been found to be over-represented in epilepsy with eyelid myoclonia. This gene encodes transcriptional regulation and not ion channels, giving a different perspective for understanding cortical excitability and PPR. CHD2 variants are also associated with photosensitivity in common epilepsies (56). An association between NEXMIF and SYNGAP1 in epilepsy with eyelid myoclonia was also observed (36).
There is a long list of gene and locus variants related to GGE, PME, other neurologic disorders and chromosomal abnormalities in which photosensitivity is present (47; 120). In their 2023 publication, Sourbron and colleagues found a pathogenic variant in GABRG2 (c.1287G> A p.(Trp429Ter)), which has been linked to photosensitive and generalized epilepsy as well as self-induced seizures (164). This variety of genotypes and phenotypes with the same or different gene raises significant difficulties for the interpretation of the results of these studies, and more research has to be done in these cases.
Localization and pathophysiology of photosensitivity. Photosensitivity results from functional abnormalities in the cortical mechanisms that control the response to strong visual stimulation (199). It is now well documented that the visual cortex is the primary site of epileptogenesis in occipital photosensitivity and pattern seizure sensitivity. This may also be true for the onset of photically induced generalized seizures in syndromes of idiopathic generalized epilepsy.
The pathophysiology of human photosensitivity has gone through several stages. Initially, the predominant view was that it is “centrencephalic” (generalized epilepsy) with the nonspecific thalamocortical reticular system activated from the lateral geniculate body (16; 58). This view, which dominated the literature at that time, was mainly based on the findings that photoparoxysmal responses are usually synchronous and generalized. The first clear evidence of the occipital origin of photoparoxysmal responses came from our studies of photically induced occipital spikes often preceding generalized photoparoxysmal responses (137; 123; 123).
Evidence that occipital spikes are preferentially elicited in photosensitive patients documents the primary or exclusive role of the occipital cortex in the initiation of photically induced seizures. This may be the only cortical region involved in occipital photosensitive seizures or the initial trigger zone of generalized photosensitive epilepsy. The visual stimuli eliciting occipital spikes depend on the number of flashes of light per second (or the number of pattern image changes per second), spatial frequency, orientation, contrast, and the line width ratio (79). All these factors indicate that the visual cortex is involved, and this is the earliest site at which integration occurs.
The primary role of the visual cortex in photosensitive epilepsy has been confirmed with elaborate documentation of pattern sensitivity, mainly in patients with photically induced seizures (197; 195; 19; 149). This has revealed many aspects of pattern seizure susceptibility and its pathophysiology:
|
• The seizures are triggered in the visual cortex. |
Generalized seizures can occur if normal excitation of visual cortex involves a "critical mass" of cortical area with synchronization and subsequent spreading of excitation from the occipital lobe trigger.
Present knowledge on pathophysiology of epileptic photosensitivity points to two types of mechanisms--mediated by the magnocellular and parvocellular systems--that contribute either synergistically or independently to elicit a photoparoxysmal response (92). Generalized photoparoxysmal responses and intermittent photic stimulation-induced occipital spikes appear to be generated independently by the parvocellular and magnocellular visual systems, respectively (78).
Color sensitivity depends on two mechanisms: one related to color modulation, intervening at low frequencies and the other dependent on single-color light intensity modulation and related to white light sensitivity that is activated at higher frequencies (140).
In epilepsy eyelid myoclonia, as opposed to other photosensitive epilepsies, eye closure is more potent than photic stimulation as a triggering factor (65; 136; 162).
However, eye closure requires the presence of light, and it is entirely ineffective in darkness. Another intriguing feature is that some patients may manifest with both features of photosensitivity and fixation-off sensitivity, which have opposing characteristics. It is possible that in patients with Jeavons syndrome, the alpha-rhythm generators malfunction and both the magnocellular and parvocellular systems are functionally disturbed (135). See the article for eyelid myoclonia for further discussion.
According to one study, the coexistence of paroxysmal eyelid movements, photoparoxysmal EEG responses, increased blinking, and epileptic eyelid myoclonia in patients with visual-sensitive reflex seizures suggests an underlying dysfunction involving cortical-subcortical neural networks of a system epilepsy (24).
Failure of inhibitory or excitatory mechanisms or both? The intermittent photic stimulation flash time-locked occipital spikes appear on the descending arm of the P100 component of the visual-evoked response (VER) (137; 123; 123).
It was postulated to be a failure of postsynaptic inhibition. However, the findings that “the P100 VER component is enhanced in photosensitive patients and that valproate slightly reduces its amplitude while occipital spikes are unaffected” were interpreted as evidence of “at least normal, if not supranormal, post-inhibitory potentials,” which suggests that the occipital spikes represent an excitatory phenomenon rather than a failure of inhibition (79), a view that was later challenged (05).
In a report, the effect of IPS at a common activating frequency (ie, 20 Hz) on motor cortex excitability was assessed by means of transcranial magnetic stimulation in 15 photosensitive patients with idiopathic generalized epilepsy (170). Nineteen normal subjects of similar age and sex acted as controls. After the resting motor threshold was measured, the corticomotor excitability was studied in two conditions randomly delivered, during IPS (5 s) at 20 Hz and without IPS. Motor evoked potentials were recorded from the right first dorsal interosseous muscle. The following parameters were determined: the cortical silent period, the short-latency intracortical inhibition at the interstimulus interval of 3 and 4 ms, and the intracortical facilitation at interstimulus interval of 12 and 14 ms. It was found that IPS at 20 Hz is significantly shortening the cortical silent period in normal subjects, whereas no significant changes were detected in patients. The resting motor threshold was significantly higher in patients than controls, as expected by the concurrent antiepileptic treatment. Other corticomotor excitability measures were unaffected. Thus, it was confirmed that IPS has a weak influence on the motor cortical output in patients. The authors concluded that the loss of the normal shortening of the cortical silent period, otherwise present in healthy subjects in response to IPS, may have a possible protective nature (170).
Brigo found that transcranial magnetic stimulation (TMS) on the primary visual cortex caused an increase in the rate of phosphenes, even at lower levels of stimulation (22).
Another report found altered recovery from inhibitory repetitive transcranial magnetic stimulation in subjects with photosensitive epilepsy. Visual responses recovered more quickly in the stimulated hemisphere, and disinhibition persisted in the contralateral side of photosensitive subjects (20). These findings collectively suggest that there is a hyperexcitability of the occipital cortex.
Perry and colleagues have found evidence for increased visual gamma responses in photosensitive epilepsy (142). A sustained gamma (30 to 70 Hz) oscillation induced in the occipital cortex by high-contrast visual stimulation has been well characterized in animal local field potential recordings and in healthy human participants using magnetoencephalography (MEG). The spatial frequency of a static grating stimulus that gives maximal gamma is also that most likely to provoke seizures in photosensitive epilepsy. The authors used MEG to study visual responses induced by grating stimuli of varying contrast and size in 12 patients with photosensitive epilepsy and two matched control groups--one with epilepsy but no photosensitivity, the other healthy controls. They used a beamformer approach to localize cortical responses and to characterize the time-frequency dynamics of evoked and induced oscillatory responses. A greater number of patients with photosensitivity had particularly amplitude gamma responses compared to controls. Formal statistical testing failed to find a group difference. One photosensitive patient, tested before and after sodium valproate, had a peak gamma amplitude when drug naive over four times larger than the group mean for controls; this high amplitude was substantially decreased after treatment with sodium valproate. There was no difference in the frequency of the sustained gamma response between the three groups. It was concluded that altered power, but not frequency, in induced cortical responses to a static grating stimulus may be a characteristic of photosensitive epilepsy. The failure to find a group difference on statistical testing may have been due to a wide intersubject variability and heterogeneity of the photosensitive group. A high amplitude response would be in keeping with previous evidence of altered contrast gain and increased spatial recruitment in photosensitive epilepsy (142).
However, continued studies using MEG and EEG in patients with photosensitivity compared to a control group indicate that such activity can function as a noninvasive biomarker for increased excitability of the visual system or occipital cortex (81; 08; 169).
Multimodal imaging documented a functional link between the circuits that trigger the posterior alpha rhythm and visual sensitivity (75; 183), a link that was also suggested for fixation-off sensitivity (135). Vaudano and colleagues investigated (A) the hemodynamic correlates of the spontaneous alpha rhythm, which is considered the hallmark of the brain resting state, in photosensitive patients and in people without photosensitivity and (B) the whole-brain functional connectivity of the visual thalamic nuclei in the various populations of subjects under investigation (183). Forty-four patients with epilepsy and 16 healthy control subjects underwent an electroencephalography-correlated functional magnetic resonance imaging study, during an eyes-closed condition. The following patient groups were included: (1) genetic generalized epilepsy with photosensitivity, 16 subjects; (2) genetic generalized epilepsy without photosensitivity, 13 patients; and (3) focal epilepsy, 15 patients. For each subject, the posterior alpha power variations were convolved with the standard hemodynamic response function and used as a regressor. Within- and between-groups second level analyses were performed. Whole brain functional connectivity was evaluated for two thalamic regions of interest, based on the hemodynamic findings, which included the posterior thalamus (pulvinar) and the medio-dorsal thalamic nuclei. Genetic generalized epilepsy with photosensitivity demonstrated significantly greater mean alpha-power with respect to controls and other epilepsy groups. In photosensitive epilepsy, alpha-related blood oxygen level-dependent signal changes demonstrated lower decreases relative to all other groups in the occipital, sensory-motor, anterior cingulate, and supplementary motor cortices. Coherently, the same brain regions demonstrated abnormal connectivity with the visual thalamus only in epilepsy patients with photosensitivity. These findings indicate that the cortical-subcortical network generating the alpha oscillation at rest is different in people with epilepsy and visual sensitivity. This difference consists of a decreased alpha-related inhibition of the visual cortex and sensory-motor networks at rest. These findings represent the substrate of the clinical manifestations (ie, myoclonus) of the photoparoxysmal response (183).
Structural MRI in photosensitive patients shows mild frontal and occipital cortical thickness, and diffusion tensor tractography reveals increased connectivity between the occipital cortex and supplementary motor cortex in juvenile myoclonic epilepsy, especially in those with photosensitivity (186). However, functional MRI studies detected activation related to PPR in extraoccipital regions, such as the parietal, premotor, supplementary cortex, and subcortical areas. These results point out abnormal networks in photosensitive idiopathic generalized epilepsies. During an fMRI of an accidental seizure, Moeller observed an increase in the BOLD signal in the superior colliculi, thalamus, and occipital cortex (117). This increase was associated with decreased activation in frontoparietal regions.
Bartolini found that the putamen plays an important role in promoting synchronization between visual and motor regions during PPR in patients with juvenile myoclonic epilepsy and that thalamic activation is essential for generalized PPR (09).
Impaired white matter integrity. In a study, diffusion tensor imaging data from MRI brain scans were collected from eight photosensitive patients and 16 gender- and age-matched nonepileptic controls using a SIEMENS Trio 3.0-Tesla scanner (46). Compared with the control subjects, the corpus callosum of patients had significantly lower fractional anisotropy values, indicating abnormal white matter in the corpus callosum of patients.
A review by Koepp shows that there may be abnormal connections in networks that include the thalamus and basal ganglia in the visually induced seizures of photosensitive patients (101). These connections may link visual activation and motor response. Additionally, H215O-PET studies show a significant increase in cerebral blood flow in subcortical and cortico-subcortical pathways of photosensitive patients, which suggests that these structures are involved in photosensitive epilepsies.
Neurotransmitters. A selective dopaminergic mechanism in human epileptic photosensitivity has been postulated (145; 144). Apomorphine, a dopamine receptor agonist, blocked photoparoxysmal responses in patients with idiopathic generalized epilepsy, and this effect was not modified by naloxone, a specific opiate antagonist, thus, suggesting that apomorphine acts on cerebral dopaminergic receptors. Conversely, apomorphine did not block spontaneous generalized spike-wave discharges in patients with non-photosensitive idiopathic generalized epilepsy.
Pathophysiology of photosensitive epilepsy in animals. Photosensitive papio-papio baboons have long been used as an animal model of photosensitive epilepsy (114; 118). They suffer from intermittent photic stimulation-induced myoclonus and have a natural predisposition to “nonepileptic myoclonus,” which is not accompanied by electrical discharges or seizures. Clinical manifestations do not show any signs of localized origin of the epileptogenic processes, and the photoparoxysmal responses are also bilateral and synchronous. However, experimental data document that the origin of photoparoxysmal responses and seizures is in the motor cortex. Photosensitive baboons also show a different pattern of activation and inhibition in blood-flow PET studies compared to normal controls, suggesting involvement of specific cortical-subcortical networks in photosensitivity (173; 172). However, studies in ferret visual cortex support a cortical etiology of pattern sensitivity (158).
In their study, Koepp and colleagues provide more details regarding ictogenic mechanisms and networks involved in photic-induced seizures (102).
Three different groups can be distinguished from an epidemiological point of view: (1) patients who experience clinical visual-induced seizures; (2) patients who have seizures and PPR; (3) people who have PPR but without clinical seizures.
The reported figures vary significantly according to reviews of existing evidence (79; 41; 134; 184), and they are highly dependent on the age and sex of the population studied, criteria for normality, and definition of EEG abnormal responses (206). The numbers may be underestimated for those with minor and infrequent seizures who do not have GTCS. There are differences in geographic distribution, with European countries having a higher incidence of PPR (10% to 15%) compared to Asian (0.6% to 1.7%) or African countries (1.6% to 3.9%) (121; 113; 160). These studies reveal that the conditions of the IPS and its duration play a crucial role in evoking a PPR (160).
Clinical photosensitivity. Clinical photosensitivity describes patients who experience clinical visual-induced seizures. Clinical photosensitivity probably affects 1 in 4000 of the population (5% of patients with epileptic seizures); two thirds are women (video game induced seizures occur more often in men); and the onset has a peak age at 12 to 13 years (79).
In a well performed demographic study in Great Britain, the annual incidence of cases of epilepsy with generalized photoparoxysmal responses on their first EEG was conservatively estimated to be 1.1 per 100,000 (representing approximately 2% of all new cases of epilepsy). When restricted to the age range 7 to 19 years, the annual incidence rose to 5.7 per 100,000 (approximately 10% of all new cases of epilepsy presenting in this age range) (147). This means that photosensitivity is found in 2% of patients of all ages presenting with seizures and 10% of patients presenting with seizures in the age range 7 to 19 years (147).
Another demographic study of the same group of authors was on seizures induced by electronic screen games (146). Of 118 patients who had a first seizure while playing an electronic screen game during two 3-month periods, three groups were identified: (Group A) 46 patients for whom there was thought to be a definite causal relationship (type 4 photoparoxysmal response); (Group B) 25 patients for whom there was a probable causal relationship (types 1 to 3 photoparoxysmal response, clinical evidence of photosensitivity, subsequent recurrent seizures on repeat exposure to electronic screen games, or occipital spikes in the resting electroencephalogram; and (Group C) 47 patients for whom there was no apparent causal relationship. The number of patients in Group C did not exceed that expected by the chance occurrence of two common events (playing electronic screen games and incidence of epilepsy). Most (103/118) of the patients were in the age range of 7 to 19 years. Within this age group, the annual incidence of first seizures triggered by playing electronic screen games (Groups A and B combined) was estimated to be 1.5/100,000.
In Japan, approximately 1% of people under the age of 18 years had seizures after watching a Pocket Monster cartoon in 1997 (85; 54). However, some had migraines, visual distortions, nausea and motion sickness, or other nonseizure symptoms, and more than half the children who experienced a previous convulsion had a history of a seizure induced by television (85).
In Panayiotopoulos studies, (1) clinical photosensitive epilepsy was found in 15 (3.4%) of 442 patients with onset of one or more afebrile seizures between birth and 15 years of age (130); (2) idiopathic photosensitive occipital lobe epilepsy was found in 11 (0.7%) of 1,550 adult and child patients with epilepsy (129); and (3) eyelid myoclonia with absences happened in about 3% of adults with epilepsy and 13% of those with idiopathic generalized epilepsy with absences (65).
EEG photosensitivity in patients with epileptic seizures. These patients have seizures with photoparoxysmal responses. The prevalence of EEG photosensitivity is probably 5% amongst patients with clinically evident epileptic seizures (79).
EEG photosensitivity is significantly higher in (a) pediatric than adult patients with epilepsy, (b) females than males, and (c) those with generalized rather than focal epilepsies. It is well documented that photoparoxysmal responses with or without clinical photosensitivity are associated with greatly variable types of epilepsy, mainly juvenile myoclonic epilepsy (30%), Dravet syndrome (70%), and Unverricht-Lundberg disease (90%) (202; 154; 06; 79; 193; 72; 167; 112; 184; 115).
Idiopathic generalized epilepsies represent 15% to 20% of persons with epilepsy, and among them, juvenile myoclonic epilepsy has the highest rate of photosensitivity. According to an ILAE paper about idiopathic generalized epilepsies, photosensitivity can be found with an appropriate protocol in up to 90% of patients with juvenile myoclonic epilepsy and frequent visually induced seizures. Photosensitivity is also found in other idiopathic generalized epilepsies like juvenile absence epilepsy but rarely in induced seizures (82).
Other rare syndromes, such as epilepsy with eyelid myoclonia, which represents 1.2% to 2.7% of cases in specialized centers, are highly photosensitive at 85%; POLE (0.7% of children with epilepsy) is photosensitive by definition (166). In Dravet syndrome (6.5/100,000 live births), photosensitivity occurs in 15% of cases (207). In progressive myoclonic epilepsies, especially in Unverricht-Lundberg disease, photosensitivity is as high as 90% (153).
EEG photosensitivity in normal subjects. Photoparoxysmal responses were found in 1.4% to 11.6% of normal school-aged children and in 0.35% to 2.4% of normal Air Force candidates (88; 95; 121; 160).
For such normal people with EEG photoparoxysmal responses, the likelihood to develop epilepsy is very low. In reports, the average prevalence of EEG photoparoxysmal responses (of any type, posterior or generalized) was 7.6% in healthy children aged 1 to 16 years, but only 3% of them developed seizures to the age of 20 years (45). In Silva’s study of 909 students, only one developed seizures (159). In other studies, none of the normal subjects with photoparoxysmal responses developed epileptic seizures over a follow-up period of 6 to 12 years (184). However, it may be possible that “asymptomatic photosensitive subjects” have unnoticed minor reflex seizures triggered by stimuli encountered in daily life.
By definition, visually sensitive epilepsy has a provocative factor, and avoiding it can be the principal measure to prevent visually induced seizures. Although it is not possible to completely avoid visually induced seizures daily, measures can be taken to reduce the risk at different levels. For instance, some stimuli characteristics can be avoided, such as red flashes, lights brighter than 20 candelas per square meter, and frequencies between 3 and 60 Hz (77; 196; 53). At the individual level, it is possible to avoid exposure to flickering lights in a nightclub or while watching TV close to the screen: the room should be well lit, decrease the duration of exposure, and avoid binocularity by covering one eye. All these precautions are especially important when playing video games. Also, it is recommended to modify the software settings on social media platforms to prevent videos from playing automatically. Due to the increased number of visual stimuli and the age of more susceptible individuals, it can be challenging to implement such measures, and, thus, advice to families plays a critical role in prevention, especially when there is a family history of photosensitivity. Educational institutions must also consider visual sensitivity safety, especially with the increased use of computers and videos as learning tools.
Technology can also help reduce the risk of visually induced seizures. Flat screen plasma and liquid crystal screens that refresh at 100 Hz seem to have less risk than older screens. The use of screen filters to reduce brightness can also be helpful.
Certain countries, including the United Kingdom, Japan, Russia, and Italy, have put in place regulations to minimize the risk of broadcasting materials that can trigger seizures. The Web Content Accessibility Guidelines (WACG) 2.0, which include photosensitivity seizure protection, are used as the standard for government and business accessibility to websites (38; 53).
The first step in the differential diagnosis is to distinguish between epileptic seizures and nonepileptic paroxysmal attacks, including psychogenic nonepileptic seizures, which are among the three major neuropsychiatric disorders (134). The workup for diagnosis should focus on a detailed history of precipitating factors. EEG with IPS can help investigate photosensitivity or induce psychogenic nonepileptic seizures (69).
Nonepileptic paroxysmal movements of the eyelids can occur in individuals with generalized photosensitive epilepsy. These movements can be misinterpreted as absence seizures but can be easily distinguished by EEG monitoring. There is often a family history of eyelid movements (29).
Other reflex seizures triggered by sensory, emotional, or cognitive stimuli can be mistaken for visually induced seizures, requiring thorough polygraphic recording for accurate diagnosis (53).
Migraine and occipital epilepsies have similar symptoms. Headaches can occur before seizures in both conditions, and migraines can have visual auras. Visual stimuli can trigger both conditions and may cause autonomic symptoms. Drawing the auras can be helpful, particularly in young children who have difficulty describing them. The auras may include lights, colored spots, and visual hallucinations in occipital seizures, whereas in migraines, they are usually more linear or zigzag and less colorful (166).
Epilepsies cannot be diagnosed on the basis of an epileptiform response to flicker alone. This EEG finding occurs in asymptomatic subjects (especially children) in several forms of epilepsy and with different seizure types. Moreover, some patients with pattern sensitive epilepsy may not be sensitive to flash intermittent photic stimulation.
Photosensitivity may also occur in severe myoclonic epilepsy of infancy (Dravet syndrome), or with degenerative gray matter encephalopathies, such as Lafora disease, Unverricht-Lundborg disease, Kufs disease, the neuronal ceroid lipofuscinoses, and others collectively known as the progressive myoclonus epilepsies in which photosensitivity at low flash frequencies is typical. These syndromes are associated with photic cortical reflex myoclonus, and the patients also have clear-cut action myoclonus.
Generalized seizures and EEG abnormalities induced by visual stimulation are conventionally differentiated from idiopathic photosensitive focal seizures with typical secondary generalization, but detailed clinical and EEG studies may be needed to make this distinction, and it should be recalled that the initial stimulus activates occipital lobe structures in both types.
A thorough medical history is the most powerful tool to diagnose visually sensitive epilepsies as any other type of epilepsy.
The most important test for confirming the diagnosis is EEG with IPS and pattern stimulation. IPS is particularly effective in generalized epilepsies (14; 21).
The objective is to confirm photosensitivity and its association with ictal events by determining the presence of PPR. The video-EEG allows the recording of minor events, like eyelid or limb jerks, that may escape without it.
EEG intermittent photic stimulation procedures. Intermittent photic stimulation (IPS) techniques and stroboscopes has differed significantly in various publications and departments. A standardized protocol proposed by Kasteleijn-Nolst Trenite enables reproducible results and inter-laboratory comparisons in EEG research (92).
Intensity, frequency, and duration of the IPS are the most significant determinants of the response. An abnormal photoparoxysmal response is more likely to occur with light of high intensity, frequency of mainly 12 to 20 Hz, and longer trains of IPS. Combining light IPS with appropriate geometric patterns makes it much more potent than diffused or white light. Distance and ambient light mainly influence the intensity of the photic stimuli.
Binocular is far more effective than monocular stimulation. This is why it is recommended that photosensitive patients cover one eye when in epileptogenic environmental photic conditions such as nightclubs.
Stimulating central vision (fixating on the light source) is much more potent than stimulating peripheral vision. This is because photosensitivity is mainly mediated through central vision and fixation. This is also the reason for the recommendation that, in IPS testing, “a central marker on the diffuser to aid fixation is specified, as photosensitivity is unlikely to be demonstrable in the eyes-open state unless the stimulator is in central vision.”
The state of the eyes during IPS is probably the most significant internal factor that modifies the response to IPS (124; 131); eye closure (closing of the eyes while IPS continues) is by far the most potent.
To ensure the effectiveness of the test, there are some important methodological considerations to keep in mind.
|
• The procedure needs to be explained to the patient. | |
|
• IPS should be performed in a dimly lit room with a lamp at a 30 cm distance from the nasion, and the patient should look at the lamp center to stimulate their central vision. | |
|
• The patient should be tested under different conditions of eyelids: open, closed, and closing. The most powerful stimuli are achieved by closing the eyes at the beginning of stimulation. | |
|
• Eyes closed are more susceptible to light than eyes open, probably due to a diffusion effect of light and the red filter created by the eyelids. | |
|
• Each frequency should be stimulated for 5 to 10 seconds, with an interval of 5 to 7 seconds between each frequency. A frame of frequencies from 1 to 60 Hz is recommended to be tested in a crescent order of 1 – 2 – 8 – 10 – 15 – 18 – 20 – 25 – 40 – 50 – 60 Hz. | |
|
• If a photoparoxysmal response is observed at any frequency, the stimulation should be stopped. After a 10-second pause, the test resumes beginning from 60 Hz and decreasing frequencies until a photoparoxysmal response is observed again (92). | |
|
• The sensitivity limit is defined as the lowest or highest flash rate that consistently evokes a photoparoxysmal response. The wider the photoparoxysmal response range, the more the patient is at risk of seizures in daily life (93). Previous reports suggested beginning with a frequency of 16 or 18 Hz to test photosensitivity, but this approach does not allow for the investigation of the limits of photosensitivity, and 17% of photosensitive patients do not show a photoparoxysmal response at this frequency (92). | |
|
• It is better to perform IPS at the end of the test because a baseline of possible discharges and a period from hyperventilation are needed. | |
|
• Failure to achieve maximal provocative IPS may produce false-negative results in the testing of patients for photosensitivity. However, prolonged photic stimulation that may expose the patient to a seizure should be avoided, especially in patients with high risks of seizures. |
EEG abnormalities in photosensitive patients. The resting EEG of patients with visual reflex seizures can be normal or frequently (20% to 30%) shows eye closure–related paroxysms occurring within 1 to 3 seconds after closing the eyes. These are usually brief EEG paroxysms lasting for 1 to 4 seconds and having similar features to those elicited by intermittent photic stimulation for each individual patient. They disappear if eye closure occurs in total darkness.
Photoparoxysmal responses are broadly categorized as:
Generalized spike or polyspike waves. They are of higher amplitude in the anterior regions, but onset, particularly if patterned intermittent photic stimulation is employed, is often with occipital spikes. They are highly associated (90%) with clinical photosensitivity, particularly if they outlast the stimulus train.
Generalized photoparoxysmal responses often (60%) associate with clinical events such as jerks, impairment of cognition, or subjective sensations, but their detection may require video-EEG.
Posterior (temporoparieto-occipital with occipital emphasis) spike or polyspike-waves. This is the mildest form of photoparoxysmal response and does not spread to the anterior regions. It consists of occipital spikes, polyspikes, or slow waves mixed with small, larval spikes.
Occipital spikes are often time-locked to the flash with a latency of approximately 100 ms, coinciding with the positive P100 of the visual-evoked response (123).
Half the patients with posterior photoparoxysmal responses have epileptic seizures (spontaneous or photically elicited or both).
The responses to IPS were categorized into four types by Doose:
|
• Type I with spikes within the occipital rhythm |
Only in Type IV is there agreement about its pathological significance in epilepsy (30). Other responses, such as photomyoclonic, are nonspecific findings reported in normal controls (0.3%) and are not considered cerebral responses (62). They are muscle activity that appears in the frontal-central EEG electrodes. They occur only when the eyes are closed and are inhibited by opening the eyes. Provocation of photomyoclonic responses requires very high-intensity IPS with the stroboscope positioned very close to the eyes. They are unlikely to occur when IPS is applied at recommended levels. Clinically, they manifest with predominant jerking of the facial muscles, especially around the eyes (eyelid fluttering), a phenomenon called photomyoclonus. The occipital spikes are often time-locked to the flash with a latency of approximately 100 ms, coinciding with the positive peak of the visual-evoked response (123).
To increase agreement among observers, Meritam Larsen proposed a version implemented with the Standardized Computer-based organized Reporting of EEG (SCORE), which considers the patient's baseline condition and the relation of the response with the stimuli.
|
1. Posterior-stimulus-dependent response | |
|
a. Limited to the stimulus train | |
|
3. Activation of preexisting epileptogenic area | |
|
a. Limited to the stimulus train | |
|
| |
Risk of seizures associated with photoparoxysmal responses. During routine EEG, the risk of seizure induction during IPS is low, with only 0.04% of the 5383 patients studied experiencing GTC seizure (194).
Warning. Responses can be greatly attenuated or abolished by some antiepileptic drugs, especially valproate, and this must be considered when interpreting the EEG for follow-up reasons and for decisions on antiseizure drug withdrawal.
Testing of pattern-sensitive patients. Although most patients sensitive to patterns experience PPR in IPS, a definitive diagnosis of pattern-sensitivity requires specific stimuli not commonly used in clinical settings. Studies indicate that certain patterns, particularly large gratings with high contrast, are more likely to trigger PPR than smaller ones. Additionally, changes in red-blue colors tend to elicit more PPR than other color combinations (81).
However, the documentation of pattern sensitivity requires specific testing with appropriate pattern presentations. Pattern sensitivity depends on the spatial frequency, orientation, brightness, contrast, and size of the pattern. An optimally epileptogenic pattern consists of black-and-white stripes of equal width and spacing.
The most epileptogenic patterns and their spatial/directional relations to the eyes have been defined by Wilkins and Binnie as follows (195; 19):
|
(1) An optimally epileptogenic pattern consists of black-and-white stripes of equal width and sharp contour (a square-wave luminance profile). | |
|
(2) The image must be well focused, and, if the subject has a refractive error, an appropriate correction must be worn. | |
|
(3) Spatial frequency is critical; this is the number of cycles of the pattern (pairs of dark and light stripes) per degree of visual angle. For most subjects without a refractive error, a spatial frequency of two to four cycles per degree is the most epileptogenic. Thus, each stripe should subtend 7.5 to 15 minutes of the arc at the eye. | |
|
(4) The orientation of the lines rarely affects epileptogenicity, except in astigmatic patients. | |
|
(5) In a susceptible subject, EEG activation is not usually seen at a luminance below 10 cd/m2 although exceptions exist. For the purposes of testing, the space-averaged luminance should be at least 200 cd/m2. The Michelson contrast (difference in luminance of light and dark stripes expressed as a proportion of their sum) should be more than 0.4. | |
|
(6) Binocular stimulation should be used. | |
|
(7) Pattern sensitivity, like visual acuity, depends mainly on central vision. Between a lower threshold and an upper saturation level is an approximately log linear relationship between pattern radius and discharge probability for circular patterns of up to 500 visual angle. To determine whether a subject is pattern sensitive, it is, therefore, worthwhile to use stimuli of at least this size. |
Although structural MRI is usually normal, it can be needed for differential diagnosis purposes. Some volumetric studies have found focal increases in thickness in occipital, frontal, and parietal cortices and a decrease in thickness in the temporal cortex in photosensitive patients compared to normal controls. Increased connectivity between the occipital cortex and supplementary motor areas has been shown with diffuse tensor tractography (110; 70; 186; 76).
Visual-induced seizures occur in patients of all ages of normal or abnormal neurocognitive state or with normal brain imaging. They may be a one-off event or life-long, focal or generalized, and responsive or resistant to antiseizure drug treatment. They frequently occur together with spontaneous seizures.
Epilepsy with eyelid myoclonia. Epilepsy with eyelid myoclonia, previously known as Jeavons syndrome, is a neurologic disorder characterized by three principal symptoms: eyelid myoclonia, eye closure–induced seizures or EEG discharges, and photosensitivity. Eyelid myoclonia typically involves a rhythmic and brief jerk of the eyelids accompanied by an upward deviation of the eyeballs and extension of the head. These seizures can occur multiple times per day and are triggered by either involuntary or voluntary closure of the eyes. Due to their brevity, it is difficult to detect any impairment of awareness, making them particularly challenging to recognize.
The EEG displays generalized and bilateral discharges of polyspikes and slow-wave complexes. Induction of PPR or seizures with eyelid myoclonia or absences with eyelid myoclonia can occur during IPS.
The age of onset is between 2 to 14 years old, with a peak at 6 to 8 years old. There is a clear female predominance, with a proportion of 2:1. Neurodevelopment is normal in most cases or with mild cognitive disorders, especially in highly photosensitive patients. Although in most cases, the eyelid myoclonia continues despite the antiseizure medication treatment, GTC seizure can be controlled. Nevertheless, epilepsy with eyelid myoclonia is considered a lifetime condition (162).
According to the latest classification of syndromes by the ILAE, the previously known "sunflower syndrome" is now considered a subgroup of epilepsy with eyelid myoclonia. It is characterized by especially prominent seizures induced by light and can be referred to as "epilepsy with eyelid myoclonia with prominent photic induction" (166). The term "sunflower" originated from the observation that patients tend to turn their heads towards the light source during seizure onset. Self-induced behavior has been described in syndromes related to compulsive behavior or associated with pleasant sensations. In epilepsy with eyelid myoclonia with prominent photic induction, it is still debatable whether the seizure is self-induced or if the head turning to the light is part of the seizure (164).
Photosensitive occipital lobe epilepsy (POLE). POLE, formerly called idiopathic photosensitive occipital lobe epilepsy, is a rare condition that is included in the ILAE’s new syndrome classification for self-limited focal epilepsies of childhood. It is often observed in females and characterized by seizures with involvement of the occipital lobe. These seizures are typically photic-induced and include visual symptoms, such as colored spots, loss of vision, blurring, and lights that move across the visual field. The age of onset for POLE is variable, ranging from 1 to 50 years, with a higher frequency between 4 and 17 years (166).
Childhood-onset visual epilepsy (COVE) is also included in this classification and is characterized by seizures with fixation-off sensitivity but not photosensitivity. It is important to distinguish between the two conditions based on the type of photosensitivity present. Additionally, differential diagnosis should be made with other entities, such as ceroid lipofuscinosis type 2 (166).
Other syndromes with visually sensitive seizures. There are several syndromes, including juvenile myoclonic epilepsy, Dravet syndrome, and some progressive myoclonic epilepsies like Unverricht-Lundberg disease, that are linked with PPR and seizures triggered by visual stimuli. However, it is important to note that spontaneous seizures are more common in these syndromes, and visually induced seizures are not the primary type of seizures (202; 154; 06; 79; 193; 72; 167; 112; 134; 103).
• Prevention measures are essential in the management of visually sensitive epilepsy. | |
• Lifestyle changes should be advised. | |
• Valproate is the first-line antiseizure medication in most cases. |
Avoidance, prevention, or modification of the provocative stimulus. Avoidance, prevention, or modification of the provocative stimulus may be sufficient for many patients with visual-induced seizures (79; 39; 134; 184).
Patients should adopt lifestyle measures, such as maintaining a regular sleep-wake cycle, avoiding excessive alcohol or drug intake, minimizing exposure to stroboscopic lights and screen usage, and managing psychological stress. Patients and their families must be made aware of these risks.
Those who show PPR in their EEG results across a wide range of frequencies are at a greater risk of developing visually induced seizures (203).
Patients with television-induced seizures should be advised to watch television in a well-lit room; maintain a maximum comfortable viewing distance (typically, more than 2.5 m from a 19-inch screen); use the remote control and, if necessary, approach the screen by covering one eye with their palm; and avoid prolonged watching, particularly if sleep-deprived and tired. Occlusion of one eye is also advised when photosensitive subjects are suddenly exposed to flickering lights, as in nightclubs, for example.
Patients with video game-induced seizures can often do without video games or significantly restrict the time spent playing. They should not play when sleep-deprived or tired.
Conditioning treatment or wearing appropriate tinted glasses has been recommended (198; 100; 31; 184). A commercially available blue lens, named Z1, was found to be highly effective in controlling photoparoxysmal discharges (31; 184). An optometric technique, colorimetry, enables the perceptual effects of ophthalmic tints to be evaluated subjectively, optimized, and then prescribed in tinted spectacles (195; 198). Checa-Ros found that other glasses with less restriction to light can also be effective for patients, with similar results and more applicability in daily life (34).
Management of visual-sensitive epilepsy also necessitates the development and implementation of guidelines to minimize exposure of susceptible populations to provocative stimuli (92). In fact, the Pokémon incident in Japan stimulated a debate on the need for regulations and protective measures for video material, particularly for television programs, to prevent seizure precipitation. Since then, official guidelines exist in some countries, notably in Japan and Europe, and have been shown to protect photosensitive subjects from the broadcasting of risky program content (176). Updated guidelines and recommendations should consider the role of parameters such as modulation depth and stimulus wavelength at provocative frequencies and the increasing availability of modern audiovisual technology that employs large screen without flicker effects but with significant changes of other variables (for instance, luminance of the screen and the separate stimulation of the two eyes). To pursue this goal, sensitization and cooperation from the industry are necessary as is the involvement of broadcasters and producers (92).
One publication presents a spatiotemporal pattern detection algorithm that can detect hazardous content in streaming video in real time (03). A tool is developed for producing test videos with hazardous content, and then those test videos are used to evaluate the proposed algorithm, as well as an existing post-processing tool that is currently being used for detecting such patterns. To perform the detection in real time, the proposed algorithm was implemented on a dual core processor, using a pipeline/parallel software architecture. Results indicate that the proposed method provides better detection performance, allowing for the masking of seizure inducing patterns in real time (03).
The risk of seizures evoked by 3D video displays or virtual reality headsets in children appears to be very low, even for those with EEG photosensitivity (182).
Prophylactic antiseizure medication. Due to the higher number of stimuli and exposure to them in modern society, avoiding provocative stimuli is not always possible. Patients who experience seizures as absences that are difficult to recognize and as tonic-clonic seizures need to be treated with antiseizure medications.
The choice of an antiseizure drugs depends on their specific efficacy on the type or types of reflex and spontaneous seizures and the particular epileptic syndrome (134). This is no different from treatment of spontaneous seizures of the same type.
In photosensitive and pattern-sensitive patients and in those with syndromes of generalized epilepsies, valproic acid, levetiracetam, and lamotrigine are the main antiepileptic drugs to recommend in that order of efficacy. Valproic acid controls all types of seizure in more than 80% of patients (134; 185). Levetiracetam controls all types of seizure, with well-established efficacy in EEG and clinical photosensitivity (98; 39); it is more efficacious in myoclonic and GTCS than absence seizures. A publication by Smith and colleagues also confirmed that valproic acid is the first-line drug, with levetiracetam or lamotrigine, in epilepsy with eyelid myoclonia (163). However, due to the drug's side effects and teratogenic potential, it is limited in its use in women of childbearing age. Lamotrigine also appears to be effective (64), but it may exaggerate jerks. Ethosuximide and lamotrigine may be particularly useful in absence seizures when valproate is ineffective or undesirable. Clonazepam is effective in photically or spontaneously induced myoclonic seizures (but is ineffective against and may exaggerate GTCS). Most of the other antiepileptic drugs (ie, carbamazepine, gabapentin, oxcarbazepine, phenytoin, pregabalin, tiagabine, and vigabatrin) are contraindicated because they are ineffective (ie, they induce side effects without providing any therapeutic benefit in addition to depriving patients of appropriate treatment).
Human photosensitive epilepsy models have been used as proof of principle drug trials for epilepsy and can be useful as early and informative indicators in anti-epileptic drug discovery and development (98; 97; 204; 74). Photosensitive patients are exposed to intermittent photic stimulation, and the reduction in sensitivity to the number of standard visual stimulation frequencies is used as an endpoint.
Patients with self-induced visual-induced seizures are difficult to treat and may need psychiatric or behavioral intervention (119). The attacks may be pleasurable, and there is clear secondary gain for some patients (177). On anecdotal evidence, fenfluramine (a serotonin-releasing drug) has been recommended for the treatment of self-induced epilepsy in combination with valproate (33).
The outcome of treatment is dependent on the type of epilepsy syndrome. In cases of idiopathic generalized epilepsies, the prognosis is favorable, and most of them respond to antiseizure medications (82). However, in juvenile myoclonic epilepsy, the continuation of PPR is a factor associated with seizures persisting over long-term follow-up (188). The sensitivity to light tends to disappear with age, but it can also continue to any age. In cases of visually sensitive epilepsy, a wide range of responses can be observed, varying from resolution with age and seizure-free, like in POLE, to pharmaceutical resistance, as seen in Dravet syndrome (166).
Most cases of visually sensitive epilepsy occur during childhood or adolescence but can persist into adulthood, posing a risk to women of childbearing age. For this reason, it is recommended to avoid the use of valproic acid, which is the first-line drug in treating visually sensitive epilepsy, and instead use other alternatives like levetiracetam or lamotrigine. Exposure of the fetus to valproic acid increases the likelihood of cognitive impairment and autistic traits (180; 181). It is essential to counsel women of childbearing age about birth control options and planning for pregnancy.
All contributors' financial relationships have been reviewed and mitigated to ensure that this and every other article is free from commercial bias.

Alicia Bogacz
Dr. Bogacz of the Institute of Neurology at the University of the Republic in Montevideo, Uruguay, has no relevant financial relationships to disclose.
See Profile
Solomon L Moshé MD
Dr. Moshé of Albert Einstein College of Medicine has no relevant financial relationships to disclose.
See ProfileNearly 3,000 illustrations, including video clips of neurologic disorders.
Every article is reviewed by our esteemed Editorial Board for accuracy and currency.
Full spectrum of neurology in 1,200 comprehensive articles.
Listen to MedLink on the go with Audio versions of each article.
MedLink®, LLC
3525 Del Mar Heights Rd, Ste 304
San Diego, CA 92130-2122
Toll Free (U.S. + Canada): 800-452-2400
US Number: +1-619-640-4660
Support: service@medlink.com
Editor: editor@medlink.com
ISSN: 2831-9125
Epilepsy & Seizures
Jan. 20, 2025
Epilepsy & Seizures
Jan. 09, 2025
Epilepsy & Seizures
Jan. 09, 2025
Epilepsy & Seizures
Dec. 23, 2024
Epilepsy & Seizures
Dec. 19, 2024
Epilepsy & Seizures
Dec. 03, 2024
Epilepsy & Seizures
Dec. 03, 2024
Epilepsy & Seizures
Dec. 02, 2024